Case Report, J Liver Disease Transplant Vol: 13 Issue: 1
A Severe Gilbert Syndrome
Dina Neto1*, Macieira F1, Ribeiro Miranda G2, Lobo M1, Oliveira Mendes S1 and Andrade F1
1Department of Medicine, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos, Portugal
2Department of Anatomic Pathology, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos, Portugal
*Corresponding Author: Dina Neto,
Department of Medicine, Hospital Pedro
Hispano, Unidade Local de Saúde de Matosinhos, Portugal
E-mail: dinaneto19@hotmail.com
Received date: 03 February, 2024, Manuscript No. JLDT-24-126767;
Editor assigned date: 05 February, 2024, Pre QC No. JLDT-24-126767 (PQ);
Reviewed date: 19 February, 2024, QC No. JLDT-24-126767;
Revised date: 26 February, 2024, Manuscript No. JLDT-24-126767 (R);
Published date: 04 March, 2024, DOI: 10.4172/2325-9612.1000253
Citation: Neto D, Macieira F, Miranda RG, Lobo M, Mendes OS, et al. (2024) A Severe Gilbert Syndrome. J Liver Disease Transplant 13:1.
Abstract
Introduction: The hyperbilirubinemias are a group of diseases characterized by alterations in the bilirubin metabolism pathway. Gilbert syndrome is a disease characterized by mild hyperbilirubinemia caused by mutations in the UGT1A1 gene. It is a benign condition. Alpha 1 Anti-Trypsin (AAT) deficiency is an inherited disease characterized by mutations in the serpin 1 gene. There are no described cases of hepatic disease by the combination of Gilbert syndrome with benign mutations of the serpin 1 gene.
Case study: A 70-year-old woman with obesity, former smoker, dyslipidemia, and pre-diabetes, presented in the emergency department after three episodes of hematemesis. At admission, she had mild anemia (10.3 g/dL). Analytical reassessment showed a decrease in hemoglobin (Hb 7.7 g/dL) and the upper digestive endoscopy showed esophageal varices. The hepatic biopsy showed cirrhosis. After a deep etiological study and the development of persistent mild cholestasis and hyperbilirubinemia, the only alterations found were a homozygous A(TA)7TAA mutation on UDPglucuronosyltransferase (UGT1A1) gene and heterozygous Glu288Val (Pi*S/M) mutation on serpin 1 gene.
Conclusion: With this clinical case, we want to show that a combination of benign mutations may cause an addition of dysfunctions and promote the development of a hepatic severe disease.
Keywords: Cirrhosis; Inherited hyperbilirubinemias; Alpha 1
antitrypsin deficiency
Introduction
The hyperbilirubinemias are a group of diseases characterized by alterations in the bilirubin metabolism pathway. These can be divided into unconjugated or conjugated hyperbilirubinemia. In the adult population, most cases (55%) of acute hyperbilirubinemia are caused by intrahepatic disorders, including viral hepatitis, alcoholic liver disease, and Drug-Induced Liver Injury (DILI). The remaining 45% of cases are extrahepatic and include gallstone disease, hemolysis, and malignancy [1].
Gilbert Syndrome is a benign genetic condition of unconjugated hyperbilirubinemia and the prevalence is about 8% globally. In the majority of cases, it does not affect liver function or cause liver damage. It is characterized by defective bilirubin conjugation due to the existence of UDP glucuronosyltransferase qualitative defect. Homozygosity or heterozygosity composts mutations of promoter A(TA)6TAA of the gene UGT1A1 (located on chromosome 2) are the mutations most common [2-4].
Alpha1-Anti Trypsin (AAT) is a proteinase synthesized in the hepatocytes, intestinal mucosa, pulmonary alveolar cells, neutrophils, macrophages, and the cornea. Its primary role is to protect lung tissue against attack by the neutrophil elastase. The serpin 1 gene is located on chromosome 14 and is the gene responsible for producing AAT [5,6]. The normal variant of the gene serpin 1 is Pi*MM. Pi*S indicates a mild deficiency of AAT, Pi*M a moderated deficiency, and Pi*Z, are the mutations that do a null activity of AAT. AAT deficiency is an autosomal recessive disorder, where the lungs and the liver are the organs the most affected [7]. The two alleles that are most commonly associated with liver disease, are homozygosity of Z or M alleles. PI*MZ (normal*null activity) may develop liver disease, especially with another hepatic insult [6,8].
Case Presentation
We present a 70-year-old woman, who is a retired journalist, with obesity grade II (38 Kg/m2); ex-smoker of 35 pack-years, dyslipidemia (last study in 2020 with Low-Density-Lipoprotein Cholesterol (LDL-C): 72 mg/dL; Total Cholesterol (TC): 134 mg/dL; High-Density-Lipoprotein Cholesterol (HDL-C): 34 mg/dL and triglycerides: 157 mg/dL), treated with high-intensity statin; prediabetes (last study in 2020 with HbA1C 6,2%), treated with metformin and depressive disorder controlled with trazodone and fluoxetine.
In November 2020, she presented to the Emergency Department (ED) with new episodes of hematemesis. Prior to that, she had no admissions to ED. A nasogastric tube was inserted and a considerable amount of dark blood was drained. The initial blood work-up investigation is demonstrated in Table 1.
| Blood workup investigation | Result | Normal range |
|---|---|---|
| Hemoglobin (g/dL) | 7.7 | Dec-16 |
| Mean Globular Volume (MGV) (fL) | 91.2 | 80-100 |
| Mean Corpuscular Hemoglobin (MCH) (pg) | 30.5 | 26-34 |
| Red Cell Distribution Width (RDW) (%) | 14.9 | 11.5-14.5 |
| Leucocyte count (Χ103/µL) | 9.45 | 04-Nov |
| Platelet count (Χ103/µL) | 181 | 150-400 |
| Serum creatinine (mg/dL) | 0.9 | 0.6-1.1 |
| Serum urea (mg/dL) | 87 | 21-43 |
| Aspartate aminotransferase (U/L) | 47 | May-34 |
| Alanine aminotransferase (U/L) | 31 | <55 |
| Total bilirubin (mg/dL) | 0.9 | 0.2-1.2 |
| Gamma-glutamyl transferase (U/L) | 49 | Sep-36 |
| Alkaline phosphatase (U/L) | 74 | 40-150 |
| Albumin g/dL | 3.9 | 3.4-4.8 |
| INR | 1.4 | - |
| C-reactive protein (mg/L) | 2.6 | <5 |
| Lactate Dehydrogenase (LDH) (U/L) | 287 | 125-220 |
| Nt pro brain natriuretic protein (Nt pro BNP) (pg/mL) | 206.9 | <125 |
| Ferritin (ng/mL) | 91.91 | 4.63-204 |
| B12 vitamin (pg/mL) | 219 | 180-382 |
| Folats (ng/mL) | 2.9 | 2.34-18.99 |
Table 1: Initial blood work-up investigation.
Intravenous fluids were started and an urgent Oesophago Gastro Duodenoscopy (OGD) was performed, which showed esophageal varices bleeding. A Computed Tomography (CT) reported a heterogeneous liver with image characteristics of cirrhosis.
A hypertensive upper gastrointestinal bleeding in a patient without a previous diagnosis of liver disease was assumed. An endoscopic and medical treatment with terlipressin, pantoprazole, and carvedilol, was done with good results and the patient stayed in the medical ward for further investigation. Ceftriaxone was started for prophylaxis of Spontaneous Bacterial Peritonitis (SBP).
An extensive etiologies investigation was done, with the exclusion of alcohol, drugs, and food not cooked consumption. Other investigations are shown in Table 2.
| Infections | |
| A hepatitis Serology | Immune |
| B hepatitis Serology | Not immune; without infection |
| C Hepatitis Serology | Negative |
| Polymerase Chain Reaction (PCR) for E Hepatitis | Negative |
| HIV Serology | Negative |
| Autoimmune disease | |
| (Gama globulins) | Normal |
| (C3 and C4 proteins) | Normal |
| Protein electrophoresis | Normal |
| Myelo Peroxidase Anti-Neutrophil Cytoplasmic Antibody (MPO-ANCA) | Normal |
| Proteinase 3 (PR3)-ANCA | Normal |
| Anti-Nuclear Antibodies (ANAs) | 1:80, with a mottled pattern |
| Anti-Mitochondrial Antibodies (AMAs) | Negatives |
| Anti-Liver-Kidney Microsomal Antibodies (LKM-Ac) | Negatives |
| Anti-Smooth-muscle antibodies | Negatives |
| Anti-liver cytosol type I Antibody | Negative |
| Anti-Soluble Liver Antigen Antibodies (SLA-Ac) | Negatives |
| Several liver-antigens autoantibodies | Negatives |
| MAFLD | |
| Insulin (N 2.2 – 25 U/L) | 6.4 U/L |
| Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (N<2) | 1.9 |
| LDL-C (N<100 mg/dL) | 77 mg/dL |
| Triglycerides (N<150 mg/dL) | 87 |
| HbA1C | 5.90% |
| Others etiologies | |
| Alfa-fetoprotein | Normal |
| Alfa-1-antytripsin (N: 84-200 mg/dL) | 211 |
| Serum ceruloplasmin | Normal |
Table 2: Extensive etiological investigation.
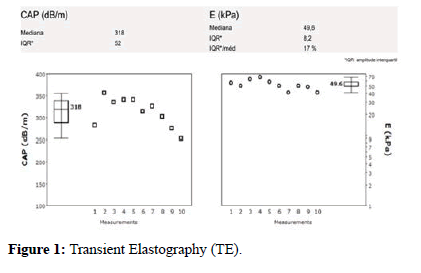
Transient Elastography (TE) was also organized and showed a Liver Stiffness (LS) of 49.6 KPa, and Inter Quartile Range (IQR): 8.2 compatible with CLD and portal hypertension (Figure 1).
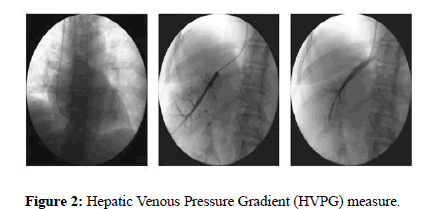
A liver catheterism was performed, which revealed sinusoidal portal hypertension with a Hepatic Venous Pressure Gradient (HVPG) of 14 mm Hg (Figure 2).
A transjugular liver biopsy confirmed the diagnosis of cirrhosis, without inflammation, steatosis, or steatohepatitis (Histologic score Metavir F4) (Figure 3).
The patient remained stable until discharge. Decompensated cirrhosis of unknown cause was diagnosed. A follow-up with the hepatology team was arranged.
At the follow-up, mild cholestasis with a 2-fold Superior Normal Limit (SNL) of alkaline phosphatase and gamma-glutamyl transferase, and a sustained mild non-conjugated hyperbilirubinemia were noted.
After the exclusion of hemolysis, an intrahepatic cholestasis genetic study was conducted, that revealed homozygosity to A(TA)7TAA mutation on UDP-glucuronosyltransferase (UGT1A1) gene, compatible with Gilbert Syndrome. In addition to this, a heterozygosity to Glu288Val (Pi*S/M) mutation on serpin 1.
Given these findings, it was assumed that the cause for the decompensated cirrhosis of this patient could be a combination of metabolic factors and benign mutations, whose combinations maybe can promote the development of severe disease and deterioration of liver function.
At the last hepatology consultation in November 2022, the patient did not have further episodes of acute decompensated cirrhosis. The last laboratory investigation showed Hb: 11.5 g/dL; total bilirubin: 1.9 mg/dL; gamma-glutamyl transferase: 61 U/L; alkaline phosphatase: 125 U/L. Last OGD showed enlarged oesophageal varices that were treated with elastic ligation (Table 3).
| Follow-up investigation | Results | Normal range |
|---|---|---|
| Hemoglobin (g/dL) | 11.5 | Dec-16 |
| Aspartate aminotransferase (U/L) | 39 | May-34 |
| Alanine aminotransferase (U/L) | 28 | <55 |
| Total bilirubin (mg/dL) | 1.9 | 0.2-1.2 |
| Gamma-glutamyl transferase (U/L) | 61 | Sep-36 |
| Alkaline phosphatase (U/L) | 125 | 40-150 |
| Albumin g/dL | 4 | 3.4-4.8 |
| INR | 1.1 | - |
| Upper digestive endoscopy | Big esophageal variceal: Performance of elastic ligation | - |
Table 3: Follow-up investigation
Results and Discussion
Successful management of cirrhosis often depends on identifying the cause and treating it. In patients where the etiology remains unknown (such as this case), clinical management may be difficult. These patients must be closely monitored with frequent blood tests and the choice of investigations is guided by the medical history [1,2]. As mentioned before, Gilbert Syndrome in most cases, does not affect liver function or cause liver damage. The mutation is needed to confirm the diagnosis but may not be sufficient for hyperbilirubinemia to occur. Other defects in bilirubin uptake, or perhaps other mutations may be necessary for biochemical or clinical expression of the mutation [3]. Moreover, the clinical expression of AAT deficiency when a patient is heterozygous to a mutation, especially when one of the alleles is Pi*M (normal allele) occurs only with another hepatic insult [5,6]. Thus, this illustrates the likely cause for the clinical picture of our patient. She has got Pi*S/M alleles on the serpin 1 gene and a homozygous to A(TA)7TAA mutation on UDPglucuronosyltransferase (UGT1A1) gene, compatible with Gilbert Syndrome. Normally, each mutation does not lead to liver damage, because (Pi*S/M) creates a mild AAT deficiency, and A(TA)7TAA mutation responsible for Gilbert Syndrome, in most cases does not cause problems. So, we hypothesize that the combinations of different liver defects, in a patient with obesity, dyslipidemia, and prediabetes all of them risk factors for the development of liver disease could explain the accumulative damage and the subsequent development of cirrhosis in this patient. After a literature search, this is the first case that showed the relevant pathological effects of these mutations.
Conclusion
This case report was a big challenge and an open mind because the patient was having a combination of benign genetic alterations that were together and enhanced by metabolic syndrome, which can explain the development of cirrhosis. This fact highlights the importance of follow-ups for patients with CLD: Not only can it delay the progression of the disease, but it may also find the cause of the cryptogenic CLD and choose the best medical treatment. The authors consider that this case report contributes to a better understanding of the big world of liver diseases.
References
- Fargo MV, Grogan SP, Saguil A (2017) Evaluation of jaundice in adults. Am Fam Physician 95(3):164-168.
- Erlinger S, Arias IM, Dhumeaux D (2014) Inherited disorders of bilirubin transport and conjugation: New insights into molecular mechanisms and consequences. Gastroenterol 146(7):1625-1638.
- Memon N, Weinberger BI, Hegyi T, Aleksunes LM (2016) Inherited disorders of bilirubin clearance. Pediatr Res 79(3):378-86.
- Wagner KH, Shiels RG, Lang CA, Seyed Khoei N, Bulmer AC (2018) Diagnostic criteria and contributors to gilbert's syndrome. Crit Rev Clin Lab Sci 55(2):129-139.
- Strnad P, McElvaney NG, Lomas DA (2020) Alpha(1)-Antitrypsin Deficiency. N Engl J Med 382(15):1443-1455.
- Patel D, Teckman JH (2018) Alpha-1-antitrypsin deficiency liver disease. Clin Liver Dis 22(4):643-655.
- De Seynes C, Ged C, De Verneuil H, Chollet N, Balduyck M, et al. (2017) Identification of a novel alpha1-antitrypsin variant. Respir Med Case Rep 64-67.
- Conde B, Costa F, Gomes J, Lopes AP, Mineiro MA, et al. (2023) Expert perspectives on the management of alpha 1-antitrypsin deficiency. Acta Med Port 36(1):49-54.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi