Research Article, J Immunol Tech Infect Dis Vol: 12 Issue: 3
A Screening Strategy to Prevent Hospital Acquired Covid -19 in Peak Pandemic Period
Jaya Garg1*, Jyotsna Agarwal1, Mohammad Saquib1, Ashish Verma1, Anupam Das1, Manodeep Sen1 and MriduSingh2
1Department of Microbiology, Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India
2Department of General Medicine, Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India
- *Corresponding Author:
- Jaya Garg
Department of Microbiology,
Ram Manohar Lohia Institute of Medical Sciences,
Lucknow,
India;
E-mail: jaya_bhu@rediffmail.com
Received date: 30 September, 2021, Manuscript No. JIDIT-23-43479;
Editor assigned date: 05 October, 2021, PreQC No. JIDIT-23-43479 (PQ);
Reviewed date: 19 October, 2021, QC No. JIDIT-23-43479;
Revised date: 26 July, 2023, Manuscript No. JIDIT-23-43479 (R);
Published date: 23 August, 2023, DOI: 10.4172/2329-9541.1000350
Citation: Garg J, Agarwal J, Saquib M, Verma A, Das A, et al. (2023) A Screening Strategy to Prevent Hospital Acquired COVID-19 in Peak Pandemic Period. J Immunol Tech Infect Dis 12:3.
Abstract
Objective: Health Care Worker (HCW) who is consistently at higher risk for Severe Acute Respiratory Syndrome Corona Virus 2 (SARSCoV-2) infection can possibly transmit the virus to vulnerable patients and other co-workers. The study is aimed to determine seroprevalence of SARS CoV-2 IgG antibody among risk group of HCW during peak pandemic period and to plan a screening strategy for early identification and isolation of HCW for safety of both HCW and exposed community.
Study setting: Hospital.
Study design: This prospective cross sectional study was conducted in North India between August-October 2020 (Peak period of pandemic). Recruited HCW grouped into high risk and low risk and were tested for the presence of SARS-CoV-2 IgG antibodies using architect automated analyser.
Data collection methods: Self-administered questionnaire were given to HCW for sociodemographic, clinical and laboratory tests results analysis related to COVID-19.
Principal findings: Out of 264 HCW, 36 (13.6%) HCW tested positive for SARS CoV-2 IgG antibodies. Seroprevalence was 14.7% in low risk group while 13.2% among high risk group. Serosurvey could detect antibody in 47.3% HCW which were either negative by COVID-19 RTPCR or were never tested owing to absence of clinical symptoms. SARS-CoV-2 IgG antibody were absent in 39% previously COVID-19 positive HCW.
Conclusion: Equal seroprevalence in both the groups of HCW during peak of pandemic is suggestive of community transmission in India and robust infection control policy of hospital. Also, after analysing pros and cons of both serological and molecular tests, we conclude that there is need of multiprong approach with serial diagnostic screening of COVID infection in health care worker which should include both RTPCR and serological test.
Keywords: SARS-CoV-2 IgG antibody; Health Care Worker(HCW); Seroprevalence; Health policy; COVID-19; Peak pandemic
Introduction
The on-going COVID19 pandemic; caused by Severe Acute Respiratory Syndrome Corona Virus 2 (SARSCoV2) was declared as pandemic on 11.03.2020 by World Health Organization (WHO). Since then it has affected globally, approx 103 million cases and 2.2 million deaths by 30.01.2021. During this entire period, Health Care Worker (HCW) has worked consistently in higher risk for COVID-19 infection as compared to other professionals. Being part of front line workers several HCWs have been infected with the Severe Acute Respiratory Syndrome Corona Virus 2 (SARSCoV-2) and have lost their lives worldwide during current pandemic. On 02.09.2020 WHO reported that 570,000 HCW were infected and 2500 dead due to SARS CoV-2 infection in United States of America. The WHO director general stressed on World patient safety day, 17 September 2020 that health worker safety is a priority for patient safety and informed that “thousands of health workers infected with COVID-19 have lost their lives worldwide” [1].
In a prospective cohort study conducted through the COVID symptom study smart phone application at United Kingdom and United States of America on comparison of COVID-19 infection among general community and frontline HCWs it was reported that compared with the general community, frontline HCWs had an adjusted hazard ratio of 11.6 (95% CI: 10.9 to 12.3) for reporting a positive test. Similar data has been reported in infectious disease outbreak previously, during the Ebola virus outbreak in Africa HCWs comprised 4.0% of all cases, 20 to 30 times higher than the general population. Similarly during SARS epidemic in 2004 HCWs comprised 20%-40% of cases. The data on nation by nation number of HCW infected with SARS CoV-2 and associated mortality is not clear as most countries do not make the data publicly available. A study was performed among members of the infectious diseases international research initiative found that although there were differences among the 37 countries that joined the survey; the median of the HCW deaths in 100,000 per population of the country was 0.05. HCW mortality per 100,000 was highest in Mexico (0.9), followed by Azerbaijan (0.44) and Italy (0.35). This data may vary from place to place as there are many issues related to under reporting by several countries and also due to inherent problems of molecular diagnostic tests for COVID-19 [2].
Establishing the true prevalence of COVID-19 infection among HCW is vital as they can possibly transmit the virus to vulnerable patients and other co-workers. Further this wills also gauzes the adequacy of infection control procedures being followed. A recent meta-analysis showed estimated overall seroprevalence of SARSCoV- 2 antibodies among HCWs as 8.7% (95% confidence interval 6.7%-10.9%). Higher sero prevalence has been reported from North America (12.7%) compared with Europe (8.5%), Africa (8.2) and Asia (4%). Further it has been seen that till now the studies on seroprevalence of HCWs worldwide has been done during the beginning of pandemic, however seroprevalence changes considerably during entire course of pandemic and to evaluate true burden of any disease the seroprevalence should be studied during peak of infection. Thus this study was planned with aim of studying seroprevalence of SARS CoV-2 IgG antibody among health care worker during peak of infection among high risk and low risk group of HCW [3].
Materials and Methods
This prospective cross sectional study on HCWs was conducted at a tertiary care medical institute, Lucknow, India from 01.08.2020 to 31.10.2020 during COVID-19 pandemic peak. 264 HCW were recruited in this study and were grouped into high risk and low risk category. High-risk group included HCW posted in COVID hospital/ wards, COVID diagnostic laboratory, fever clinic, holding area and emergency area whereas low risk group included HCW who never performed duty in COVID hospital/laboratory and were mainly administrative and clerical office staff. Written informed consent was collected by all HCW and the study was approved by the institute ethical committee [4].
Eligible health care workers were provided with a self-administered questionnaire to capture socio demographic characteristics, job profile, COVID-19 symptoms such as fever, cough, breathlessness, sore throat, loss of smell etc, exposure history to laboratory-confirmed COVID-19 cases and history of COVID-19 illness. Compliance to adherence with recommended Infection Prevention and Control (IPC) measures was also checked. The questionnaire was modified from the protocol. “Assessment of potential risk factors for 2019-novel corona virus infection among HCW in a healthcare setting”, published by WHO.
2 mL of venous blood was collected from each participant using aseptic precautions. Samples from high risk group HCWs were collected 28th day after their duty in respective areas and samples from low risk group HCWs were collected randomly. Serum samples were tested for the presence of SARS-CoV-2 specific IgG antibodies on the Abbott Architect automated analyser using the Abbott SARS-CoV-2 IgG assay as per the manufacturer's instructions. This assay has a sensitivity of 100% and specificity of 99.6%. As a part of quality control all positive and 10% negative serum samples were re-tested using the same assay [5].
Results
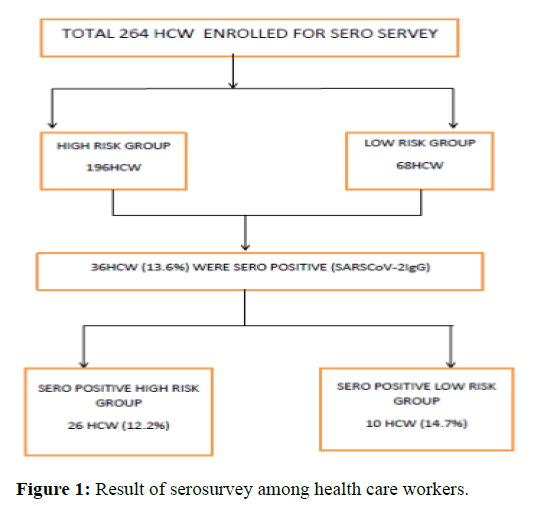
A total of 264 health care workers were enrolled in study between August to October 2020. Of these 134 were male and 130 female (Ratio M:F-1.03). One hundred and ninety six HCWs were posted in high COVID risk areas (COVID hospital-50, COVID laboratory-38, fever clinic-45, hospital emergency and holding area-63) and 68 HCWs were posted in low risk areas (Administrative office-10, non- COVID laboratory technician-20, paramedical staff in non-COVID hospital-38). Among high risk HCW the mean age was 32 year (25 year-45 year) and was lowered as compared to mean age in low risk HCW 45 year (29 year-58 year) [6-9].
Of the 264 HCW included in study, 36 tested positive for the presence of IgG antibodies against SARS-CoV-2; resulting in an overall seroprevalence of 13.6%. Seroprevalence was 14.7% (10/68 HCW) in low risk group while 13.2% (26/196) among high risk group. All positive samples were rechecked using same assay. Out of thirty six seropositive health care worker 19 HCW (52.8%) gave history of previous respiratory illness along with positive COVID-19 RTPCR report; rest 17 (47.3%) gave neither history of respiratory disease nor were they tested for COVID-19 by RTPCR [10-13].
46 HCW gave previous history of COVID RTPCR test positivity. Among them SARS-CoV-2 antibody developed only in 28 (61%) HCW and rest 18 (39%) did not show any immune response. Among 28 HCW who were both SARS CoV IgG and COVID-19 RTPCR positive; 20 HCW (71.5%) showed mild symptoms of COVID-19, 6 HCW (21.4%) had moderate infection while 2 HCW (7.1%) suffered from severe infection of COVID-19. Review of hospital records all 18 SARS CoV IgG antibodies negative and COVID-19 RTPCR positive HCW showed that all of them had developed mild symptoms of COVID-19 (Table 1).
| Diagnosis | SARS CoV-2 IgG antibody positive | SARS CoV-2 IgG antibody negative | Total |
|---|---|---|---|
| COVID RTPCR positive | 28 | 18 | 46 |
| COVID RTPCR negative/unknown | 8 | 210 | 218 |
| Total | 36 | 228 | 264 |
Table 1: Correlation between seroprevalence and previous RTPCR results.
Discussion
SARS CoV-2 infection among healthcare worker is associated with disruption of patient care, risk of transmission to patients and family members, mental stress, morbidity and even mortality. Therefore, protection of HCWs from COVID-19 and early diagnosis with isolation is a worldwide priority. While reverse transcriptionpolymerase chain reaction is the gold standard for COVID-19 diagnosis among symptomatic cases, mild or asymptomatic s cases may not be reported/tested or COVID RTPCR tests may be negative in them. Here sero diagnosis using detection of SARS CoV-2 IgG antibody might be useful and seroprevalence studies may be an important tool for diagnosis of past infection among HCW [14,15].
Seroprevalence studies conducted worldwide showed prevalence ranges from 0% to 13%. Most of these seroprevalence studies were done at the start of pandemic effectively from May to June. Sero prevalence study during the peak period of pandemic will not only evaluate the maximum risk of infection in different risk group of HCW in hospital settings but also evaluate the compliance of infection control policy of a hospital.
The peak of COVID pandemic of different countries is tabulated in where in European countries the new COVID cases/day were maximum during the months of October, November and December. In United States and South Africa two peaks in infection graphs were appreciated, the first peak in South Africa occurred in July 2020 and in United States it occurred in November 2020 followed by second peak in January 2021 owing to emergence of new mutant SARS CoV-2 strains. In most of these countries seroprevalence data among HCW is available for sero surveys that were conducted before peak of infection, leading to false negativity (Table 2) [16-19].
| Country | Time of COVID-19 peak 14 | Seroprevalence data of HCW before pandemic peak |
|---|---|---|
| United States of America | January 2021 | 12.7% |
| South Africa | January 2021 | 8.2% |
| Germany | December 2020 | 1.6% |
| Italy | November 2020 | 3.4% |
| England | January 2021 | 6% |
| Belgium | November 2020 | 6.4% |
| India | September 2020 | 11. 94% |
Table 2: Peak pandemic period of COVID-19 infection worldwide with seroprevalence of the infection among HCW before pandemic peak.
India being the second most populous country with high population density is at high risk from COVID-19 pandemic and as per existing Indian data released by Ministry of health and family welfare, New Delhi of 1366 million Indian population; 10.7 million (0.08%) developed RTPCR confirmed COVID-19 infection. Studies of seroprevalence among HCW during the first 3 month of pandemic vary from 4.3% to 14%. A study from Kerala, South India even showed zero seroprevalence in HCW at the beginning of pandemic. The peak months of infection in India were August to October with approx. 60,000 cases/day and maximum 97,894 cases on single day were recorded on 17.09.2020.
This study was conducted when COVID-19 pandemic was at peak in India. Seroprevalence estimated in the study is 13.6% but interesting fact is that low risk group has high seroprevalence (14.7%) compared to high risk group (13.2%). Similar finding are also reported in a recent study conducted by sero surveillance group of Indian Council of Medical Research (ICMR), New Delhi at time of peak COVID-19 infection. The study reported seroprevance of 6.5% in both groups.
This may be attributable to adherence of high risk group HCW to Infection Prevention and Control (IPC) measures. In spite of increased work load at the peak period of COVID pandemic, robust implementation of infection control protocols and strict adherence to personal protective equipment guidelines of institute had evidenced a significant role in preventing infection in HCWs and lower than that of low risk group. Low risk group can be surrogate marker of community spread and reflect the more of infection rate in community at the time of pandemic peak. Less stress full working condition along with their mobility to community showed even high seroprevalence rate compare to that of high risk group (Figure 1) [20].
Most of the studies on seroprevalence excluded the RTPCR confirmed seropositive HCW but in this study we have compared serological results of HCW with their previous history of laboratory confirmed COVID infection. Sero survey was able to detect SARS CoV-2 IgG antibody in 47.3% HCW which were either negative by RTPCR or were never tested owing to absence of any respiratory tract infection in past. Serological tests can analyze overall immune response in a population and virus-specific IgG antibodies developed after infection can stay in the blood for several weeks to months after symptom onset. Also study shows that 20% to 80% of SARS-CoV-2- positive cases are estimated to be asymptomatic, hence serostudies are helpful in detecting undiagnosed past infection and give information regarding the disease prevalence in a population. To measure true burden of risk of COVID-19 infection in HCW, routine screening strategy should be made in which serological test should be done in parallel along with RTPCR.
Health care workers who were positive previously by SARS-CoV-2 RTPCR testing had a higher seroprevalence 61.0% compared with those who did not tested negative or were not aware of their result 3.7%. Another study reported that individuals who were positive on previous SARS-CoV-2 testing had a higher seroprevalence 80.9% than those who tested negative or were not aware of their result. In our study 39% HCW in which SARS CoV2 IgG antibody didn’t develop, had mild infection which suggest that in mild cases of COVID-19 development or persistence of immune response is difficult. There are several studies documenting that antibody responses differ significantly in asymptomatic individuals and individuals with mild or severe COVID-19. Limitation of our study is that correct evaluation of molecular and serology test should be done by comparing them with gold standard test i.e., SARS-CoV-2 viral culture by enrolling HCW for parallel testing by both.
Conclusion
With the re-emergence of mutant strain of COVID-19 and recurrence of pandemic leading to multiple peaks, it is necessary to determine an strict infection prevention policy and standard protocol to determine exposure so as to prevent the spread of infection.
Serological test can detect the hidden cases of HCW infected with COVID but on the other hand molecular tests will identify those HCWs in whom immune response didn’t developed or persisted even after acquiring COVID infection. Analysing the pros and con of both tests, there is need of multi prong approach to confirm the results and reduce the rate of false-negative test results. Serial diagnostic screening policy of COVID infection in health care worker must include both RTPCR and serological testing to identify the infected HCW to prioritize them to isolate and help immensely to decrease hospital-based transmissions.
Disclaimers
None.
Source(s) of Support/Funding
None.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Ethical Issues
The work has been approved by institute ethical committee of Dr RMLIMS, Lucknow, India in which it was performed and that subjects gave informed consent to the work.
References
- Chang D, Xu H, Rebaza A, Sharma L, Cruz CSD (2020) Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med 8: 12-13.
[Crossref] [Google Scholar] [PubMed]
- Kang HM, Choi EH, Kim YJ (2021) Updates on the coronavirus disease 2019 vaccine and consideration in children. Clin Exp Pediatr 64: 327-328.
[Crossref] [Google Scholar] [PubMed]
- Xu M, Wang D, Wang H, Zhang X, Liang T, et al. (2020) COVID‐19 diagnostic testing: Technology perspective. Clin Transl Med 10: 157-158.
[Crossref] [Google Scholar] [PubMed]
- Dohla M, Boesecke C, Schulte B, Diegmann C, Sib E, et al. (2020) Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Publ Health 182: 170-172.
[Crossref] [Google Scholar] [PubMed]
- Erdem H, Lucey DR (2021) Healthcare worker infections and deaths due to COVID-19: A survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis 102: 239-241.
[Crossref] [Google Scholar] [PubMed]
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D (2021) Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: A systematic review and meta-analysis. J Hosp Infect 108: 120-134.
[Crossref] [Google Scholar] [PubMed]
- Goenka MK, Afzalpurkar S, Goenka U, Das SS, Mukherjee M, et al. (2020) Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India 68: 14-19.
[Google Scholar] [PubMed]
- Houlihan CF, Mc Gowan CR, Dicks S, Baguelin M, Moore DA, et al. (2017) Ebola exposure, illness experience and Ebola antibody prevalence in international responders to the West African Ebola epidemic 2014–2016: A cross-sectional study. PLoS Med 14: 1002300.
- Koh D, Lim MK, Chia SE (2003) SARS: Health care work can be hazardous to health. Occup Med 53: 240-241.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Sathyapalan D, Ramachandran A, Subhash K, Biswas L, et al. (2021) SARS-CoV-2 antibodies in healthcare workers in a large university hospital, Kerala, India. Clin Microbiol Infect 27: 481-483.
[Crossref] [Google Scholar] [PubMed]
- Gamage B, Moore D, Copes R, Yassi A, Bryce E, et al. Protecting health care workers from SARS and other respiratory pathogens: A review of the infection control literature. Am J Infect Control 33: 114-121.
[Crossref] [Google Scholar] [PubMed]
- Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, et al. (2021) SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020-January 2021. Int J Infect Dis 108: 145-155.
[Crossref] [Google Scholar] [PubMed]
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, et al. (2020) Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 5: 475-483.
[Crossref] [Google Scholar] [PubMed]
- Prakash O, Solanki B, Sheth JK, Joshi B, Kadam M, et al. (2021) Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: A cross-sectional study. BMJ Open 11: 044101.
[Crossref] [Google Scholar] [PubMed]
- Roy S. (2020) COVID-19 reinfection: Myth or truth? SN Compr Clin Med 2: 710-713.
[Google Scholar] [PubMed]
- Singhal T, Shah S, Naik R, Kazi A, Thakkar P (2020) Prevalence of COVID-19 antibodies in healthcare workers at the peak of the pandemic in Mumbai, India: A preliminary study. Indian J Med Microbiol 38: 461-463.
[Crossref] [Google Scholar] [PubMed]
- Haselmann V, Kittel M, Gerhards C, Thiaucourt M, Eichner R, et al. (2020) Comparison of test performance of commercial anti-SARS-CoV-2 immunoassays in serum and plasma samples. Clin Chim Acta 510: 73-78.
[Crossref] [Google Scholar] [PubMed]
- Wu D, Wu T, Liu Q, Yang Z (2020) The SARS-CoV-2 outbreak: What we know. Int J Infect Dis 94: 44-48.
[Crossref] [Google Scholar] [PubMed]
- Baldwin JM, Eassey JM, Brooke EJ (2020) Court operations during the COVID-19 pandemic. Am J Crim Justice 45: 743-758.
[Crossref] [Google Scholar] [PubMed]
- Younes N, Al-Sadeq DW, Al-Jighefee H, Younes S, Al-Jamal O, et al. (2020) Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses 12: 582.
[Crossref] [Google Scholar] [PubMed]
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi