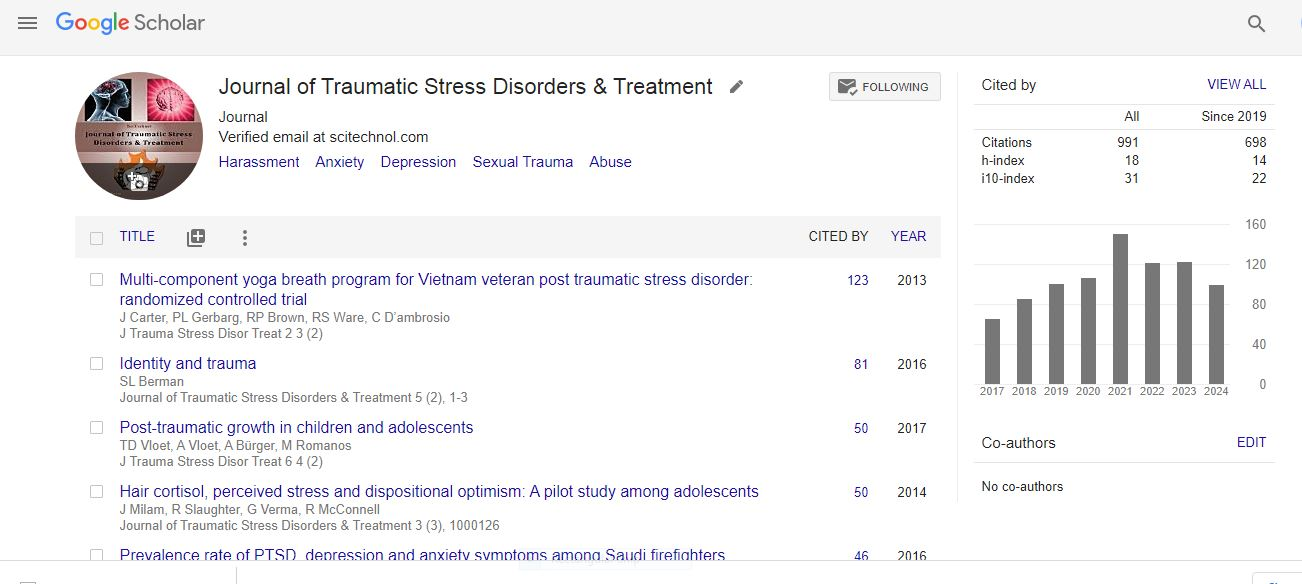
Research Article, Jtsdt Vol: 13 Issue: 5
A Pilot Investigation of the Effectiveness of the Sana Device in Management of PTSD: A Blinded Randomized Controlled Trial
Stephanie Hart1,2*, Wendy Muzzy1,3, Michelle Pompei1,3, Jeffrey Bower4, Mark Robberson4, Richard Hanbury4, Ronald Acierno1,5
1Ralph H. Johnson VA Health Care System, United States
2Lowcountry Center for Veterans Research, United States
3Military Sciences Division, Medical University of South Carolina, United States
4Sana Health Inc., United States
5Faillace Department of Psychiatry, University of Texas Health Science Center, Houston, United States
*Corresponding Author: Stephanie Hart
Ralph H. Johnson VA Health Care System and Lowcountry Center for Veterans Research, United States
E-mail: zeigls@musc.edu
Received: 24-Oct-2024, Manuscript No. JTSDT-24-150887;
Editor assigned: 25-Oct-2024, PreQC No. JTSDT-24-150887 (PQ);
Reviewed: 02-Nov-2024, QC No. JTSDT-24-150887;
Revised: 07- Nov-2024, Manuscript No. JTSDT-24-150887 (R);
Published: 15-Nov- 2024, DOI:10.4172/2324-8947.1000417
Citation: Hart S, Muzzy W, Pompei M, Bower J, Robberson M, et al. (2024) A Pilot Investigation of the Effectiveness of the Sana Device in Management of PTSD: A Blinded Randomized Controlled Trial. J Trauma Stress Disor Treat 13:5.
Copyright: © 2024 Hart S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Post-traumatic Stress Disorder (PTSD) is a chronic and debilitating condition affecting millions of Americans, particularly veterans. Repercussions of PTSD are multifaceted, encompassing psychological, physical, and social challenges. Frontline pharmacological treatments only offer limited relief and come with detrimental side effects. Psychological treatments like Prolonged Exposure (PE) or Cognitive Processing Therapy (CPT) are often poorly tolerated by patients leading to early discontinuation of treatment. There is an urgent need for additional treatment options that can be used in addition to the current pharmacological and psychological therapies for those with PTSD. The present study investigated the Sana Device, a neuromodulatory Audio-Visual Stimulation (AVS) device designed to remediate symptoms of PTSD. Methods: We conducted a randomized, parallel-arm, controlled trial with 48 Veterans age 18-65 diagnosed with PTSD who were seeking services from a Southeastern Veterans Affairs Hospital to investigate effectiveness of the Sana device plus best practices PTSD Treatment as Usual (Sana+TAU) relative to best practices Treatment as Usual (TAU) alone for treating PTSD. Participants were asked to use the Sana device with best practices TAU or continue best practices TAU alone for 28 days. Patients were assessed on PTSD symptoms (CAPS-5 & PCL-5), anxiety (GAD7), depression (PHQ-9), and quality of life (PGIC-QOL). Results: Significant improvements were evident in Sana+TAU vs. TAU for PTSD symptom severity (PCL-5 p < 0.001). In addition, there were significant improvements for the Sana+TAU arm over TAU alone for anxiety (GAD-7: p < 0.001), depression (PHQ-9: p < 0.001), and for Quality of Life (PGIC-QOL: p < 0.001). Conclusion: The Sana Device + TAU was more effective than best practices TAU alone for improving PTSD symptoms, anxiety, depression, and quality of life for patients with PTSD. The Sana device shows promise as an effective novel treatment for PTSD that is easy to use, largely free of side effects, and works alongside existing treatments.
Keywords: PTSD, Post-Traumatic Stress Disorder, PCL-5, CAPS-5, Neuromodulation, AVS, Audio Visual Stimulation, Veterans
Introduction
PTSD is a pervasive mental health condition that can develop after experiencing or witnessing a life-threatening event, like combat, a natural disaster, a car accident, or sexual assault [1,2]. PTSD can occur after a single traumatic event or from prolonged/repeated exposure to trauma [3,4]. Types of traumas associated with PTSD include war and combat, violence and abuse, and disaster and terrorism, with the most common traumas being combat exposure, witnessing among men, and rape and sexual molestation among women [5]. The National Comorbidity Survey Replication (NCS-R), conducted between February 2001 and April 2003, comprised interviews of a nationally representative sample of 9,282 Americans aged 18 years and older. PTSD was assessed among 5,692 participants, using DSM-IV criteria. The NCS-R estimated the lifetime prevalence of PTSD among adult Americans to be 6.8%. The lifetime prevalence of PTSD among men was 3.6% and among women was 9.7% [6]. Within veterans, the rate of PTSD is greater than in the general population and is estimated to be between 11-12% [7]. Survival analysis shows that more than one third of people with an index episode of PTSD fail to recover even after many years [5].
The persistence of PTSD symptoms over time highlights the severe impact this disorder can have on individuals' lives. It is a life-threatening condition that has historically been associated with suicide and related behaviors [8]. Suicidal ideation and attempts are understood to be higher in those with PTSD than in the general population and meta-analyses have estimated that the rate of death by suicide is nearly 4 times greater in PTSD patients than in the general population [9-11]. A recent retroactive cohort study used PTSD Checklist (PCL) data from 1999 to 2018 from veterans to better understand the association between PTSD severity and rate of suicide. In this study, he found that in PTSD patients with initial PCL assessments of greater than 18 had twice the rate of suicide mortality 1 month after assessment compared to those that had scores below the threshold. Additionally, patients with worsening symptoms had a 25% greater chance of mortality from suicide. Finally, this study estimated that patients that achieve remission of PTSD symptoms through treatment had a significant reduction in suicide rates [12].
The risk of suicide in those with PTSD is further exacerbated by delays in treatments and as baseline severity of symptoms increases [12,13]. This creates challenges when designing randomized controlled trials (RCTs) examining new interventions to treat moderate to severe PTSD. Due to the risk of self-harm which increases without treatment, it is unethical to use a waitlist control or sham device that would essentially delay treatment for those assigned to these arms. In addition, to examine treatments within patients with moderate to severe symptom levels, the risk of self-harm for PTSD patients not receiving an active treatment increases further as the risk of suicide increases with PTSD severity [12].
Currently available and effective treatments for PTSD have limitations in tolerability, availability, social acceptance, and cost. Front-line treatments for PTSD include trauma-focused psychotherapies, such as PE and CPT [14]. Antidepressants (SSRIs and SNRIs) are also commonly recommended for patients suffering from PTSD, but evince weaker impact [14]. Written Exposure Therapy is evidence based treatment for PTSD, but has a smaller body of research in support of its use.
While PE effectively reduces symptoms of PTSD, poor tolerance to this treatment by patients’ results in dropout from these treatments is unacceptably high. Moreover, effectiveness in well controlled clinical trials may not be representative of response in the general population [15-17] . For example, the dropout rate for clinical trials for patients beginning treatment with PE is 20% with medication trials exceeding that rate, compared to closer to 50-55% in clinical settings [18,19]. In a large cohort study on the utilization of PTSD treatments after returning from deployment in 45,462 soldiers, the median number of treatments visits were 4 in 6 months of time, 22% only ever completed one treatment visit, 24% dropped out of treatment, and only slightly more than half (52%) received minimally adequate care [20].
Furthermore, availability of PE, CPT, and WET for those with PTSD is severely limited. Current estimates show that only a third of psychotherapists have the training and certification to conduct CPT or PE [21]. In addition, literature suggests that most clinical practitioners do not employ empirically supported methods and treatments, such as PE and CPT, to treat PTSD due to unfamiliarity or not being comfortable with these approaches [22]. Within the VA it was estimated that only a third of practitioners had training to treat PTSD and that only a few hundred practitioners out of 6000 in the VA were trained to administer PE or CPT [23]. Finally, PTSD treatments are both time consuming and costly. Healthcare costs for those with PTSD pose a large burden with a higher costs per individual than coronary heart disease as well as other psychological conditions like major depressive disorder, which are thought to be one of the most burdensome and costly mental health conditions [24-26] . Taken together, this suggests that new effective interventions need to be developed that are better tolerated, more easily deployed, require less training, and can be used remotely without a clinician present than the current standard of care.
The current trial investigated a novel Audio-Visual-Stimulation (AVS) device that has demonstrated potential across several indications (See methods for a description of the device). AVS is a form of neuromodulator that has been used for performance enhancement and management of insomnia symptoms [27]. When the brain is given a stimulus through the eyes or ears, it emits a responsive electrical charge, called a Cortical Evoked Response (CER). The brain responds by synchronizing to it, a process called Frequency Following Response (FFR). FFR can be used to trigger each electrical pattern to potentially put the brain into a restful, healthy state of relaxation [28]. The Sana Device utilizes this AVS mechanism to induce FFR. Treatment with the AVS device aims to provide effective relief of symptoms, improvement in quality of life, reduction in side effects associated with current treatments, and that is adaptable to the variable nature of PTSD. The AVS program delivered by the device delivers both immediate and potentially lasting improvement for neurological and psychological disorders. The AVS program immediately encourages beneficial mental states and broadly promotes a more balanced cross-hemispheric signal. The benefits apply broadly across psychological conditions that can benefit from reductions in anxiety, poor sleep quality, and depression as well as for the symptoms of PTSD.
Methods
Study Design and Setting
This study was designed to assess the effectiveness of the Sana device when added to best practices Treatment as Usual (i.e., referral to PE, CPT, or WET without waitlist) in participants with a diagnosis of post-traumatic stress disorder (PTSD) on severity of symptoms as measured by CAPS-5. This study used a 2-arm repeated measures randomized controlled design in which participants were randomly assigned to either Sana plus best practices Treatment as Usual (Sana+TAU) or best practices Treatment as Usual (TAU).
The trial was conducted at participants’ homes with participants recruited from a southeastern VA healthcare facility. All participants signed an informed consent document prior to enrollment into the study. Sessions were offered at the clinic or via telehealth. Before the study was initiated, the study protocol was submitted to the WCG Institutional Review Board (IRB) and received approval on 29-Sep-2022.
Participants
This study enrolled adult veterans who had served, or were currently serving, in the US military; age 18 to 65 years, with a diagnosis of PTSD; and who had no medical conditions that would prevent them from safely participating in the study or using the Sana device. See (Table 1) for specific inclusion/exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 1. Having served, or are currently serving, in the US military. | 1. Pregnant, intending to become pregnant or lactating females as self-reported. |
| 2. Willing to and capable of providing written electronic informed consent prior to the conduct of any study-related procedures. | 2. History or presence of photo-sensitive epilepsy or other photo-sensitive conditions as self-reported. |
| 3. Adults, of any sex or gender, 18 to 65 years of age. | 3. History or presence of condition(s) that may affect balance, such as seizure disorders or vertigo as self-reported. |
| 4. Diagnosis of PTSD as determined by a Clinician Administered PTSD Scale for DSM-5 (CAPS-5) clinical interview or CAPS-5 severity = 25. | 4. History or presence of severe and continuous tinnitus, at investigator discretion |
| 5. Must be in good physical health based on self-report. | 5. Surgery or trauma requiring rehabilitation within the last 12 weeks as self-reported. Presence of cancer pain, acute pain following injury or other severe pain that would be anticipated to change during the course of the study, at discretion of the investigator. |
| 6. Any psychotropic drug therapy regimen must be stable (unchanging) for at least 4 weeks prior to enrollment and remain steady throughout the study. | 6. Vision impairments that affect perception of light, color, or brightness in one or both eyes, and differences in visual perception between eyes, per patient self-report. |
| 7. Willing and able to comply with the study requirements, complete study assessments, and participate at scheduled times for the duration of the study. | 7. Deafness in one or both ears, perceived differences in hearing between ears, per patient self-report. |
| 8. Able to understand, speak, and read English sufficient for the completion of study assessments. | 8. Current ear or eye infection, untreated allergies, or acute illness that may affect eyes or hearing (e.g., due to congestion), per patient self-report. |
| 9. Provision of appropriate storage and charging for study equipment in a generally safe and dry condition. | 9. Presence of inflammation or broken skin around the eyes in the area of the mask, per patient self-report. |
| 10. Presence of narcolepsy or untreated sleep apnea, per patient self-report. Note: presence of sleep apnea is permitted, so long as patients feel comfortable to use both apnea mask and Sana device in conjunction. | |
| 11. Participation in any other clinical study in which medication(s) are being delivered or have used an investigational drug or device within the last 30 days. | |
| 12. Any pending legal action that could prohibit participation or compliance in the study, per patient self-report. | |
| 13. Recent history of or current evidence of suicidal intent or active suicidal behavior based on patient self-report. Entry into the study is at investigator’s discretion. | |
| 14. Significant medical conditions or other circumstances which, in the opinion of the investigator, would preclude compliance with the protocol, adequate cooperation in the study or obtaining informed consent, or may prevent the patient from safely participating in study. | |
| 15. Employment by the investigator or the study site, with direct involvement in the proposed study or other studies under the direction of the investigator or study site, or a family member of an employee or of the investigator. | |
| 16. Use of drugs that can produce hallucinogenic effects (i.e., Ketamine or psilocybin mushrooms) within the past 4 weeks. |
Study Procedures
Individuals were recruited from a Southeastern VA Health Care System catchment area. Recruited participants completed informed consent and a baseline interview to determine inclusion/exclusion criteria, including the CAPS-5 PTSD assessment. Participants meeting inclusion/exclusion criteria were randomized to either the Sana plus best practices Treatment as Usual or best practices Treatment as Usual (PE, CPT, or WET without wait times were offered to all participants in both conditions). A decentralized trial vendor, Curavit, was used to administer remote assessments and track visits.
The treatment phase lasted 28 days. During the treatment phase, participants in the Sana arm underwent at least 2 daily sessions of device use, including a usage session immediately prior to bedtime. Additional sessions of device treatment were allowed at the participant’s discretion. During the treatment phase, participants completed telehealth visits on days 1, 3, 7, 14 and 21 by a coordinator to check on progress and adherence to expected device use.
All post-baseline assessments were administered remotely. On Day 14 and Day 28, participants completed self-report assessments. Additionally, on Day 28, participants completed a CAPS-5 assessment via a telehealth video or phone call with a VA Health Care System staff member. Assessments of adverse events (AEs), treatment acceptability, and adherence were also collected.
Each participant participated in the study for up to 49 days, including Screening and Baseline (up to 14 days), Treatment (Sana+TAU or TAU alone; 28 days), and Off-boarding (up to 7 days). Participants received assessments at Day -14 to 0 (Screening and Baseline), Day 14 (Mid-Treatment), and Day 28 (Post Treatment).
Measures
PTSD Checklist 5 (PCL-5)
The PCL-5 is a 20-item self-reported measure that assesses the 20 DSM-5 symptoms of PTSD. The PCL-5 can be used to screen individuals for PTSD as well as monitor symptom changes during and after treatment. The PCL-5 was administered at all assessment time points (Baseline, Day 14, and Day 28).
Clinician-Administered PTSD Scale for DSM-5 (CAPS-5)
The CAPS-5 is a 30-item structured interview that can be used to diagnosis and assess PTSD symptoms and severity. The CAPS-5 is administered by clinicians or trained professionals, the full interview takes 45-60 minutes to administer. The CAPS-5 was administered at Baseline and Day 28. If a CAPS-5 was on file that has been completed within 2 weeks prior to consent, that information was used to determine eligibility
General Anxiety Disorder (GAD-7)
The GAD-7 is a self-reported questionnaire for screening and measuring severity of generalized anxiety disorder. The GAD-7 has 7 questions which assess severity of GAD over the last 2 weeks. The GAD-7 was administered at all assessment time points (Baseline, Day 14, and Day 28).
Patient Health Questionnaire-9 (PHQ-9)
The Patient Health Questionnaire 9-item is a validated tool for screening, diagnosing, monitoring and measuring depression severity and scores each of the 9 Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM-IV) related criteria. The PHQ-9 was administered at all assessment time points (Baseline, Day 14, and Day 28).
Patient Global Impression of Change (PGIC - QOL)
The Patient Global Impression of Change (PGIC - QOL) is a commonly validated measure that is recommended to assess a patient’s overall change, with regards to their person and in satisfaction with their treatment, over the duration of the study. Using this scale, the participant can indicate any changes in their activity limitations, symptoms, emotions and overall quality of life, as related to their pain condition, since the start of the study (1= No change to 7=A great deal better). The PGIC - QOL was administered at Day 28.Treatments.
Treatments
Sana Device
The Sana device is an externally worn mask that physically contacts the skin of the face. The Sana device delivers Audio Visual Stimulation (AVS) in the form of coordinated pulses of light (through closed eyelids) and sound (through earphones) at targeted frequencies. The Sana device is externally communicating only and meets the definition of a “Non-Significant Risk” device.
Best Practices Treatment as Usual (TAU)
Patients assigned to either arm also received best practices TAU and were asked to maintain the use and dosage of any prescribed medications. As mentioned, best practices TAU comprised a referral to immediately begin PE, CPT, or WET. For Sana+TAU, 2 veterans started PE and 13 started WET; for TAU, the numbers were identical (i.e., 2 started PE and 13 started WET).
Data Analysis
For the CAPS-5, PCL-5, GAD-7, and PHQ-9 mean difference between groups from Baseline to Day 28 was tested for statistical significance using a Linear Mixed model with an alpha criterion of 0.05. The model had a categorical factors for group (Sana+TAU | TAU) and Visit (# of visits in the model depend on the specific outcome) as well as the interaction between Group and Visit. The model used a by-subject intercept to account for within subject correlations across visits. Linear mixed models, with a by-subject intercept, are commonly used in repeated measures trials and comparable results to Repeated Measures ANOVA. The PGIC-QOL scale was only collected at the Day-28 visit, and thus, was analyzed using a simple liner model with a factor for group. Pre-planned contrasts from within the 2-way interaction of group (Sana+TAU | TAU) and Visit (Baseline | Day 28) were examined for all outcomes, beside the PGIC-QOL, which used a pre-planned contrast examining group (Sana+TAU | TAU) at Day-28. All pre-planned contrasts used 2-tailed tests of superiority. In addition, post-hoc analyses of within group change for each outcome were conducted to provide additional context.
Final analyses were conducted in 2 populations. The Intent to Treat (ITT) population included all participants who were randomized and completed the baseline assessment. Due to the properties of the linear mixed model used in the analysis, means were automatically estimated even in the presence of missing data, thus for outcomes using the mixed model, no imputation of scores is required. For the PGIC-QOL within the ITT population, multiple imputations were used to estimate missing scores. The results for 20 imputed data sets were pooled using Rubin’s rules [29]. The Per-Protocol (PP) population included participants who were randomized, completed the Baseline and Week 4 assessments, began treatment, and did not have major any protocol deviations. For the PGIC- QOL scale, participants will have to have completed the Day 28 assessment to be included.
All analyses were conducted in the statistical programming language R [30], within the R-Studio IDE [31]. In addition, the following libraries were used; Diplyr [32], Tidyr [33], Lme4 [34], LmerTest [35], Emmeans [36], and Mice [37].
Results
Participant Flow / Dropouts
A total of 48 subjects were consented into the study. A total of 46 (23 Sana+TAU, 23 TAU) were included in the ITT analysis and a 37 (17 Sana+TAU, 20 TAU) were included in the PP analysis. Please see the Consort Chart (Figure 1) for additional details.
Demographics
Demographics for participants included in the ITT analysis are presented in Table 2.
| Arm | Gender | N | Mean (SD) |
|---|---|---|---|
| Sana+TAU & TAU | All | 46 | 41.67 (12.20) |
| Sana+TAU | All | 23 | 44.30 (12.14) |
| Female | 7 | 42.86 (14.70) | |
| Male | 16 | 44.94 (11.33) | |
| TAU | All | 23 | 39.04 (11.94) |
| Female | 6 | 43.00 (14.91) | |
| Male | 17 | 37.65 (10.89) |
Table 2: Participant Demographics
Endpoint Results
For the PCL-5, there was a statistically significant improvement the Sana+TAU arm over the TAU arm in the ITT population (Mean difference =11.1, p < 0.001) and the PP population (Mean difference = 11.7, p < 0.001). The mean change from Baseline to Day 28 for the PCL-5 for both analysis populations are shown in Figures 2 and 3. Within and between group summary statistics are presented in Table 3.
| Outcome | Population | Contrast | Estimate | SE | T-Ratio | P-Value |
|---|---|---|---|---|---|---|
| PCL-5 | ITT | Sana+TAU - TAU | 11.073 | 3.209 | 3.451 | <0.001 |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 18.522 | 2.343 | 7.905 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 7.449 | 2.193 | 3.397 | 0.001 | ||
| (Baseline - Day 28) | ||||||
| PP | Sana+TAU - TAU | 11.65 | 3.258 | 3.576 | <0.001 | |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 19 | 2.395 | 7.932 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 7.35 | 2.208 | 3.328 | 0.001 | ||
| (Baseline - Day 28) | ||||||
| CAPS-5 | ITT | Sana+TAU - TAU | 4.663 | 3.244 | 1.437 | 0.158 |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 11.847 | 2.319 | 5.109 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 7.184 | 2.269 | 3.166 | 0.003 | ||
| (Baseline - Day 28) | ||||||
| PP | Sana+TAU - TAU | 4.183 | 3.468 | 1.206 | 0.236 | |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 11.294 | 2.487 | 4.542 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 7.111 | 2.417 | 2.942 | 0.006 | ||
| (Baseline - Day 28) | ||||||
| GAD-7 | ITT | Sana+TAU - TAU | 4.778 | 1.079 | 4.427 | <0.001 |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 4.87 | 0.788 | 6.181 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 0.092 | 0.737 | 0.125 | 0.901 | ||
| (Baseline - Day 28) | ||||||
| PP | Sana+TAU - TAU | 4.782 | 1.103 | 4.334 | <0.001 | |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 4.882 | 0.811 | 6.018 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 0.1 | 0.748 | 0.134 | 0.894 | ||
| (Baseline - Day 28) | ||||||
| PHQ-9 | ITT | Sana+TAU - TAU | 4.106 | 1.32 | 3.109 | 0.003 |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 8.3 | 0.946 | 8.773 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 4.194 | 0.921 | 4.553 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| PP | Sana+TAU - TAU | 4.369 | 1.306 | 3.346 | 0.001 | |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 8.647 | 0.937 | 9.233 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 4.278 | 0.91 | 4.7 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| PGIC-QOL | ITT | Sana+TAU - TAU | 2.026 | 0.513 | 3.952 | 0.017 |
| (Baseline - Day 28) | ||||||
| Sana+TAU | 5.026 | 0.343 | 8.856 | <0.001 | ||
| (Baseline - Day 28) | ||||||
| TAU | 3 | 0.343 | 2.918 | 0.007 | ||
| (Baseline - Day 28) | ||||||
| PP | Sana+TAU - TAU | 2.662 | 0.453 | 5.882 | <0.001 | |
| (Day 28) | ||||||
| Sana+TAU | 5.412 | 0.333 | 10.254 | <0.001 | ||
| (Day 28) | ||||||
| TAU | 2.75 | 0.307 | 2.445 | 0.02 | ||
| (Day 28) |
Table 3: Between and within group statistics for all outcomes, populations, planned between group contrasts, and post-hoc within group contrasts
CAPS-5 scores were not statistically significantly different between the Sana+TAU arm and the TAU arm in the ITT population (Mean difference =4.7, p = 0.16) and the PP population (Mean difference = 4.2, p < 0.24). Note: low statistical power insofar as at the calculated effect size of 0.41 it would take 95 participants per group, at 80% power with a 2-tailed T-Test with an alpha criterion of 0.05. The mean change from Baseline to Day 28 for the CAPS-5 for both analysis populations are shown in Figures 4 and 5. Within and between group summary statistics are presented in Table 3.
For the GAD-7, there was a statistically significant improvement the Sana+TAU arm over the TAU arm in the ITT population (Mean difference =4.8, p < 0.001) and the PP population (Mean difference = 4.8, p < 0.001). The mean change from Baseline to Day 28 for the GAD-7 for both analysis populations are shown in Figures 6 and 7. Within and between groups summary statistics are presented in Table 3.
For the PHQ-9 assessment, there was a statistically significant improvement the Sana+TAU arm over the TAU arm in the ITT population (Mean difference =4.1, p = 0.003) and the PP population (Mean difference = 4.4, p = 0.001). The mean change from Baseline to Day 28 for the PHQ-9 for both analysis populations are shown in Figures 8 and 9. Within and between groups summary statistics are presented in Table 3.
For the PGIC-QOL assessment, there was a statistically significant improvement the Sana+TAU arm over the TAU arm in the ITT population (Mean difference =2.7, p < 0.001) and the PP population (Mean difference = 2.7, p < 0.001). The mean change from Baseline to Day 28 for the PGIC-QOL for both analysis populations are shown in Figures 10 and 11. Within and between group summary statistics are presented in Table 3.
Between and within group statistical results are summarized in Table 2 below. The Estimate is the mean difference between groups for between groups contrasts and is the mean change for within group contrasts.
Safety Results
For this study there were a total of 28 AEs. Only 9 of 28 adverse events were reported to be “possibly” or “probably” related to the study device. The remaining 19 were “unrelated” or “unlikely” to be related to the study device. Of the AEs deemed to be “probably” or “possibly” related to the device 7 were considered “mild”, 2 were considered “moderate”, and none were considered “severe”. All treatment related AEs resolved with only one resulting is discontinuation of device use. From this data, it can be concluded that the Sana Device is safe and does not pose any unknown risks to end-users.
Discussion
This randomized, parallel-arm, and controlled study evaluated the effectiveness of a novel AVS device (Sana) for improving PTSD symptoms, anxiety, depression, and quality of life in 46 patients with PTSD. The results of this trial showed significant improvements in the Sana+TAU arm over TAU for PTSD symptoms (PCL-5), anxiety (GAD-7), depression (PHQ-9), and quality of life (PGIC-QOL). Indeed, considering the ITT sample, PTSD scores as measured by the PCL-5 for Sana+TAU showed a 19 point decrease during the 28 day period, compared to a 7 point decrease in the TAU condition. Similarly, anxiety scores on the GAD-7 showed a 5 point drop for Sana+TAU, relative to a less than 1 point drop for TAU alone. Furthermore, depression scores on the PHQ-9 were reduced 8 points in the Sana+TAU group versus 2 points in response to TAU alone. Finally, the 5 point improvement on the general quality of life measure (PGIC-QOL) was also significantly better for Sana+TAU relative to TAU (3 points). Only the CAPS-5 failed to reach statistical significance, despite the 12 point drop for Sana+TAU relative to the 7 point drop for TAU.
Considering that this small sample study was not powered for efficacy findings and considering that the comparator best practices TAU treatments (i.e., PE, CPT, WET) themselves have extremely powerful effects, the fact that all self-report measures across symptom and functioning evinced significant improvement, over and above referral to best practices treatment, is extremely encouraging. We designated the CAPS-5 as the primary outcome measure a priori, and this measure did not show a significant improvement over TAU. However, this preliminary study was knowingly underpowered at about 25 participants per group. Moreover, we compared Sana’s treatment-augmenting effects to referral to the best available PTSD interventions available, rather than to a ‘straw man’ no treatment controls condition to increase the real-world relevance of our findings. As such, statistical power limitations should not be taken lightly. Indeed, at the calculated effect size of 0.41, it would take 95 participants per group to achieve at 80% power (2-tailed t-test, alpha of 0.05).
As mentioned, both conditions included immediate referral to PE, CPT or WET, as well as continued use of any psychotropic medications already on board. As such, this test of the Sana device was in its capacity as an additive intervention. Other attempts at supplementing or altering evidence-based PTSD interventions exist, and either target treatment retention or treatment efficacy. Considering the former, studies of massed PE and CPT show promise for reducing treatment dropout [38,39] , as does including peers directly during in vivo exposure trials [40].However, utility of supplemental intervention components in increasing treatment effects, measured either in terms of PTSD symptoms or overall functioning, have been less consistent. For example, He reviewed the literature and identified 28 augmentation interventions classified according to their mechanism of action (e.g., cognitive enhancers such as rTMS or attention training; emotional distress reducers such as biofeedback training, fear extinction enhancement such as yohimbine or D-cycloserine; integrated interventions addressing sleep or social rehabilitation, etc.) [41]. These authors found that most augmentation efforts were not fruitful, and that the best augmentation interventions were those that targeted cognitive enhancers. Interestingly, Sana falls into that category. Perhaps future research based on contemporary calls for RCTs using psychedelic augmented PTSD treatments will produce fruitful results, but the legal and side effect considerations of those treatments present far more obstacles to use than an intervention such as the Sana device.
Limitations
Limitations of this study and design were evident and should be noted. To approximate real-world conditions as well as best practices standards, participants in both conditions received referral to best practices PTSD treatments in the form of PE, CPT, or WET (i.e., per their choice). However, participants were not required to engage in these treatments. While increasing generalizability, this introduces an obvious experimental confound with respect to both the type of evidence-based treatment obtained, and differential rates of evidence-based treatment actually engaged in across groups. This concern is somewhat mitigated by the fact that Sana achieved its effects in only 28 days, a period during which standard evidence-based treatments are only 25% completed, and only in the initial phases of engaging in ‘active components’ of treatment. In other words, although it would be methodologically preferable to require all participants in all groups to engage in the same evidence-based treatment either with or without Sana, observed results likely emerged prior to any impact such homogeneity of intervention would have had. Other limitations of our study are similar to those characterizing other PTSD treatment outcome research, including heterogeneity of trauma type, frequency, and time since trauma event; and lack of control over variables such as age and TBI status that may impact outcome insofar as brain changes may play a role in treatment mechanism outcomes.
Conclusion
This signal finding trial showed that the Sana Device + best practices Treatment as Usual was more effective than best practices Treatment as Usual alone for improving PTSD symptoms, anxiety, depression, and quality of life for patients with PTSD. The Sana device shows promise as an effective novel treatment for PTSD that is easy to use, largely free of side effects, and works in alongside existing treatments. Future research involving fully powered designs is now warranted.
References
- Wisco BE, Nomamiukor FO, Marx BP, Krystal JH, Southwick SM, et al (2022) Posttraumatic stress disorder in US military veterans: Results from the 2019–2020 National Health and Resilience in Veterans Study. J Clin Psychiatry, 83(2):39779.
- Gradus JL (2020) PTSD: National Center for PTSD. US Department of Veterans Affairs.
- Inoue C, Shawler E, Jordan CH, Moore MJ, Jackson CA. Veteran and military mental health issues.
- Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, et al (2012) Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 9(4):361.
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (2013) Posttraumatic stress disorder in the National Comorbidity Survey. 22-34.
- Kessler RC, Chiu WT, Demler O, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 62(6):617-27.
- Greenberg G, Hoff R (2016) Veterans with PTSD data sheet: National, VISN, and VAMC tables.
- Krysinska K, Lester D (2010) Post-traumatic stress disorder and suicide risk: a systematic review. Arch Suicide Res. 14(1):1-23.
- Cougle JR, Keough ME, Riccardi CJ, Sachs-Ericsson N (2009) Anxiety disorders and suicidality in the National Comorbidity Survey-Replication. J Psychiatr Res. 43(9):825-9.
- McCarthy JF, Bossarte RM, Katz IR, Thompson C, Kemp J, et al (2015) Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 105(9):1935-42.
- National Center for Health Statistics. Health, United States, 2016, with chartbook on long-term trends in health.
- Forehand JA, Dufort V, Gradus JL, Maguen S, Watts BV, et al (2022) Association between post-traumatic stress disorder severity and death by suicide in US military veterans: retrospective cohort study. Br J Psychiatry. 221(5):676-82.
- Krysinska K, Lester D (2010) Post-traumatic stress disorder and suicide risk: a systematic review. Arch Suicide Res. 14(1):1-23.
- Department of Veterans Affairs. Department of Defense (2017) VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder.
- Seligman ME (1995) The effectiveness of psychotherapy: The Consumer Reports study. Am Psychol. 50(12):965.
- Imel ZE, Laska K, Jakupcak M, Simpson TL (2013) Meta-analysis of dropout in treatments for posttraumatic stress disorder. J Consult Clin Psychol. 81(3):394.
- Van Etten ML, Taylor S (1998) Comparative efficacy of treatments for post‐traumatic stress disorder: A meta‐analysis. Clin Psychol Psychother. 5(3):126-44.
- Bradley R, Greene J, Russ E, Dutra L, Westen D (2005) A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry. 162(2):214-27.
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, et al (2003) Do patients drop out prematurely from exposure therapy for PTSD? J Trauma. Stress. 16:555-62.
- Hoge CW, Grossman SH, Auchterlonie JL, Riviere LA, Milliken CS, et al (2014) PTSD treatment for soldiers after combat deployment: Low utilization of mental health care and reasons for dropout. Psychiatr Serv. 65(8):997-1004.
- Covington, C (2019) Not just for soldiers: Civilians with PTSD struggle to find effective therapy.
- Becker CB, Zayfert C, Anderson E (2004) A survey of psychologists’ attitudes towards and utilization of exposure therapy for PTSD. Behav Ther. 42(3):277-92.
- Tanielian T, Farris C, Epley C, Farmer CM, Robinson E, et al (2014) Ready to serve. Community-based provider capacity to deliver culturally competent, quality mental health care to veterans and their families.
- Davis LL, Schein J, Cloutier M, Gagnon-Sanschagrin P, Maitland J, et al (2022) The economic burden of posttraumatic stress disorder in the United States from a societal perspective. J Clin Psychiatry. 83(3):40672.
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC (2015) The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 76(2):5356.
- Vigo D, Thornicroft G, Atun R (2016) Estimating the true global burden of mental illness. Lancet Psychiatry. 3(2):171-8.
- Tang HY, Riegel B, McCurry SM, Vitiello MV (2016) Open-loop audio-visual stimulation (AVS): a useful tool for management of insomnia? Appl Psychophysiol Biofeedback. 41:39-46.
- Cahn BR, Polich J (2006) Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 132(2):180.
- Rubin DB (2004) Multiple imputation for nonresponse in surveys.
- Team RC (2020) RA language and environment for statistical computing, R Foundation for Statistical. Computing.
- Team RS (2021) R Studio: integrated development environment for R.
- Wickham H, François R, Henry L, Müller K (2021) dplyr: A grammar of data manipulation (Version 1.0. 8).
- Wickham H, Vaughan D, Girlich M (2023) tidyr: Tidy messy data (Version 1.3. 0).
- Bates D (2014) Fitting linear mixed-effects models using lme4. arXiv preprint.
- Kuznetsova A, Brockhoff PB, Christensen RH (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw. 82(13).
- Lenth R, Singmann H, Love J, Buerkner P, Herve M (2019) Emmeans: estimated marginal means, aka least-squares means (Version 1.3. 4).
- Van Buuren S, Groothuis-Oudshoorn K (2011) mice: Multivariate imputation by chained equations in R. J Stat Softw. 45:1-67.
- Galovski TE, Werner KB, Weaver TL, Morris KL, Dondanville KA, et al (2022) Massed cognitive processing therapy for posttraumatic stress disorder in women survivors of intimate partner violence. Psychol Trauma. 14(5):769.
- Peterson AL, Blount TH, Foa EB, Brown LA, McLean CP, et al (2023) Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 6(1):e2249422-.
- Hernandez-Tejada MA, Bruce MJ, Muzzy W, Birks A, Macedo e Cordeiro G, et al (2024) Peer support during in vivo exposure homework increases likelihood of prolonged exposure therapy completion. Mil Psychol. 1-0.
- Metcalf O, Stone C, Hinton M, O’Donnell M, Hopwood M, et al (2020) Treatment augmentation for posttraumatic stress disorder: A systematic review. Clin Psychol. 27(1):e12310.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi