Editorial, J Food Nutri Disor Vol: 9 Issue: 3
A Perspective on Nutrition and Cancer
Amandio Vieira*
BPK Nutrition and Metabolism Laboratory, Simon Fraser University, Canada
*Corresponding Author : Amandio Vieira
BPK Nutrition and Metabolism Laboratory, Simon Fraser University, Canada
E-mail: avvieira@sfu.ca
Received: April 17, 2020 Accepted: July 20, 2020 Published: July 27, 2020
Citation: Amandio Vieira (2020) A Perspective on Nutrition and Cancer. J Food Nutr Disor 9:3. doi: 10.37532/jfnd.2020.9(3).e110
Abstract
Research into nutrition and cancer typically involves either a study of dietary factors in the context of cancer prevention, or dietary changes to moderate the effects of conventional cancer therapy. Cancer involves genetic and epigenetic changes that influence gene expression programs controlling cell growth, death, differentiation. Some of the changes leading to genomic instability can be influenced by diet and other environmental factors. In this context, a genoprotective diet may be considered as one that is low in harmful dietary factors and sufficient in protective dietary factors. Harmful factors, e.g. carcinogens, are ones that can promote cancer. Possible beneficial factors include some vitamins, minerals, and phytochemicals. The B-vitamin folate is an example of a possible protective factor; among other functions, it is involved in dTMP (DNA) biosynthesis and has a role in epigenetic control, e.g. [1-4].
The role of nutrition in the etiology of cancers is complex and not well understood; and some of the related studies are difficult to interpret. This complexity arises from different aspects of these studies: (a) Cancer is a complex group of diseases; and each one often involves a chronic, multistep pathological process characterized
by dysregulation of cell growth and death, loss of differentiation, inflammation, etc. (b) Human diet is typically also complex; it is difficult to isolate the roles of specific dietary factors-typically found in most diets-that can promote or protect against the pathological process of carcinogenesis. (c) Another category of problems lies
in the application of laboratory studies and animal model studies to carcinogenesis and human cancer prevention. Often isolated compounds are tested at relative high concentrations, i.e., outside of the food context. Moreover, there may be important differences in the way a model organism reacts to, and processes, a dietary compound in relation to human metabolism.
At a basic level, cancer development and progression typically involves one or more of the following factors: (i) damage to DNA (mutations). Reactive chemical species such as ROS can cause oxidative damage to DNA, e.g. [5,6]; and this may be exacerbated by deficiency of antioxidants. Other genetic damage may be caused
by deficiencies in nutrients such as folate (see above) or in the cells’ genetic repair systems. (ii) High-level expression of cancerpromoting genes (oncogenes) or reduced expression of protective, tumour-suppressor genes. Such expression may be modified by various dietary factors, e.g. retinoids (from vitamin A) and calciferols (vitamin D). (iii) Suppressed immunity may also contribute to the progression of cancers, and many nutrient deficiencies (e.g. zinc and vitamin A) can compromise immunity.
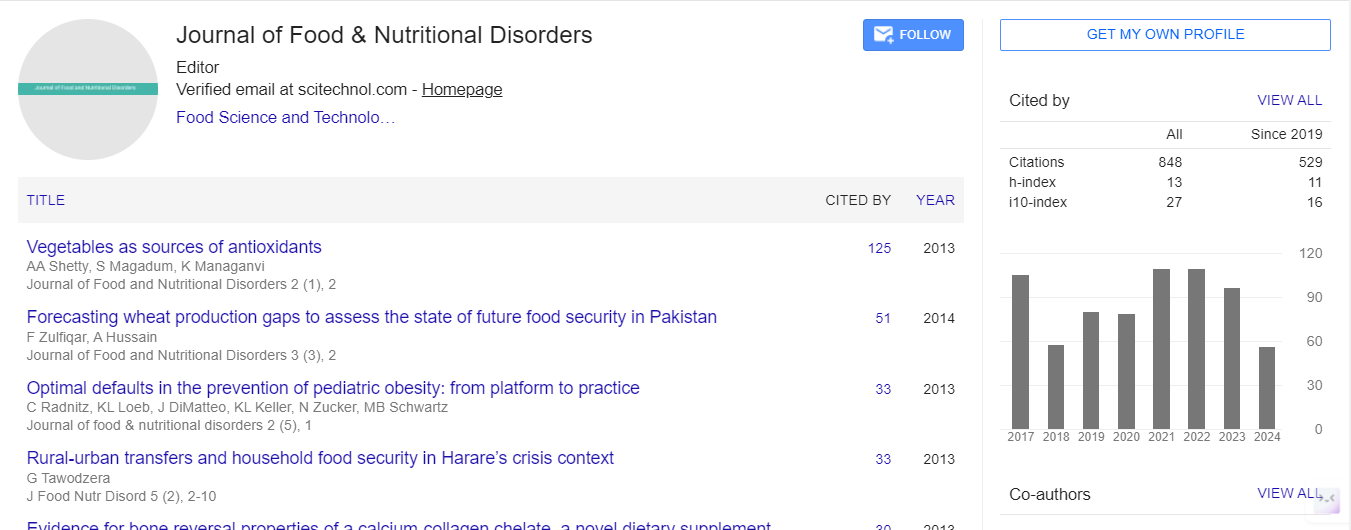
As an example of gene-diet interactions, one can consider genetically-determined DNA repair efficiency interacting with two general classes of dietary parameters: insufficient intake of a protective factor such as folate or vitamin E, and excessive intake of harmful factors in food such as heterocyclic aromatic amines and other possible carcinogens (Figure 1). An individual with efficient repair systems is likely able to better tolerate potentially damaging nutrient deficiencies, or a relatively higher level of carcinogen exposure. It has been estimated that a majority of adulthood cancers may be caused by environmental factors [7]. (Some early-onset or childhood cancers are likely much less influenced by harmful environmental factors.) There are both dietary (nutrient deficiencies and food carcinogens) and non-dietary (e.g. smoking and radiation) components to such environmental factors. At a global level, major causes of cancer include smoking, UV radiation, obesity, alcohol, infections. Putative dietary carcinogens typically rank lower (except alcohol), but their exact contribution is not well known. Possible food carcinogens may arise from (i) industrial chemicals used to treat foods, e.g., pesticides, (ii) environmental contaminants from air, soil, etc., (iii) food processing, e.g. colours, flavours, and other additives
and (iv) high temperature cooking and exposure to combustion products during cooking. Red meats, rich in heme, have been associated with increased risk of colorectal cancer [8].
Heme iron can participate in reactions that lead to oxidative damage [9]. High fat and high energy diets can increase risk of some types of cancer, e.g. [10,11]. Interestingly, a recent animal model study involving high fat diet has suggested that surgical removal of some fat deposits can decrease the development of UV-induced skin cancers [12]. Other studies have suggested benefits for bariatric surgery in some people against cancer incidence or progression, e.g. [13]. In terms of protective factors, fruits, vegetables, teas, are commonly recommended based on a large number of populationlevel studies. Plant foods have many potential beneficial factors:
dietary fibre, vitamins, and other photochemicals, as well as a relatively low caloric density. Many phytochemicals exhibit antioxidant activities in vitro, but the potential relevance of many of these activities in the body (in vivo) is not well established. Photochemicals can influence many cellular functions and thereby
Keywords: Food science, Nutrition
Editorial
Research into nutrition and cancer typically involves either a study of dietary factors in the context of cancer prevention, or dietary changes to moderate the effects of conventional cancer therapy. Cancer involves genetic and epigenetic changes that influence gene expression programs controlling cell growth, death, differentiation. Some of the changes leading to genomic instability can be influenced by diet and other environmental factors. In this context, a genoprotective diet may be considered as one that is low in harmful dietary factors and sufficient in protective dietary factors. Harmful factors, e.g. carcinogens, are ones that can promote cancer. Possible beneficial factors include some vitamins, minerals, and phytochemicals. The B-vitamin folate is an example of a possible protective factor; among other functions, it is involved in dTMP (DNA) biosynthesis and has a role in epigenetic control [1-4].
The role of nutrition in the etiology of cancers is complex and not well understood; and some of the related studies are difficult to interpret. This complexity arises from different aspects of these studies: (a) Cancer is a complex group of diseases; and each one often involves a chronic, multistep pathological process characterized by dysregulation of cell growth and death, loss of differentiation, inflammation; (b) Human diet is typically also complex; it is difficult to isolate the roles of specific dietary factors-typically found in most diets that can promote or protect against the pathological process of carcinogenesis; (c) Another category of problems lies in the application of laboratory studies and animal model studies to carcinogenesis and human cancer prevention. Often isolated compounds are tested at relative high concentrations i.e., outside of the food context. Moreover, there may be important differences in the way a model organism reacts to, and processes, a dietary compound in relation to human metabolism.
At a basic level, cancer development and progression typically involves one or more of the following factors: (i) damage to DNA (mutations). Reactive chemical species such as ROS can cause oxidative damage to DNA [5,6]; and this may be exacerbated by deficiency of antioxidants. Other genetic damage may be caused by deficiencies in nutrients such as folate (see above) or in the cells’ genetic repair systems. (ii) High-level expression of cancer promoting genes (oncogenes) or reduced expression of protective, tumoursuppressor genes. Such expression may be modified by various dietary factors, e.g. retinoids (from vitamin A) and calciferols (vitamin D). (iii) Suppressed immunity may also contribute to the progression of cancers, and many nutrient deficiencies (e.g. zinc and vitamin A) can compromise immunity. As an example of gene-diet interactions, one can consider genetically-determined DNA repair efficiency interacting with two general classes of dietary parameters: insufficient intake of a protective factor such as folate or vitamin E, and excessive intake of harmful factors in food such as heterocyclic aromatic amines and other possible carcinogens. An individual with efficient repair systems is likely able to better tolerate potentially damaging nutrient deficiencies, or a relatively higher level of carcinogen exposure.
It has been estimated that a majority of adulthood cancers may be caused by environmental factors [7]. (Some early-onset or childhood cancers are likely much less influenced by harmful environmental factors.) There are both dietary (nutrient deficiencies and food carcinogens) and non-dietary (e.g. smoking and radiation) components to such environmental factors. At a global level, major causes of cancer include smoking, UV radiation, obesity, alcohol, infections. Putative dietary carcinogens typically rank lower (except alcohol), but their exact contribution is not well known. Possible food carcinogens may arise from (i) industrial chemicals used to treat foods, e.g pesticides, (ii) environmental contaminants from air, soil, etc. (iii) food processing, e.g. colours, flavours, and other additives and (iv) high temperature cooking and exposure to combustion products during cooking. Red meats, rich in heme, have been associated with increased risk of colorectal cancer [8]. Heme iron can participate in reactions that lead to oxidative damage [9]. High fat and high energy diets can increase risk of some types of cancer [10,11]. Interestingly, a recent animal model study involving high fat diet has suggested that surgical removal of some fat deposits can decrease the development of UV-induced skin cancers [12]. Other studies have suggested benefits for bariatric surgery in some people against cancer incidence or progression [13].
In terms of protective factors, fruits, vegetables, teas, are commonly recommended based on a large number of population level studies. Plant foods have many potential beneficial factors: dietary fibre, vitamins, and other photochemical, as well as a relatively low caloric density. Many phytochemicals exhibit antioxidant activities in vitro, but the potential relevance of many of these activities in the body (in vivo) is not well established. Photochemical can influence many cellular functions and thereby modulate basic path physiological properties such as cell growth, differentiation, and death. Phytoestrogens, for example [14], can modulate steroid hormone-related actions in the body. In relation to cancer, there are also examples of photochemicals that can affect carcinogen metabolism, growth factor cell signaling, inflammation, and epigenetic regulation [15-20].
References
- Kawakita D, Matsuo K, Sato F, Oze I, Hosono S, et al. (2012) Association between dietary folate intake and clinical outcome in head and neck squamous cell carcinoma. Ann Oncol 23: 186-192.
- King WD, Ho V, Dodds L, Perkins SL, Casson RI, et al. (2012) Relationships among biomarkers of one-carbon metabolism. Mol Biol Rep 39: 7805-7812.
- Duthie SJ (2011) Folate and cancer: How DNA damage, repair and methylation impact on colon carcinogenesis. J Inherit Metab Dis 34: 101-109.
- Bistulfi G, Vandette E, Matsui S, Smiraglia DJ (2010) Mild folate deficiency induces genetic and epigenetic instability and phenotype changes in prostate cancer cells. BMC Biol 8: 6.
- Weyemi U, Dupuy C (2012) The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutat Res 751: 77-81.
- Sfikas A, Batsi C, Tselikou E, Vartholomatos G, Monokrousos N, et al. (2012) The canonical NF-κB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage. Cell Signal 24: 2007-2023.
- Hemminki K, Forsti A, Bermejo JL (2006) Gene-environment interactions in cancer: do they exist? Ann NY Acad Sci 1076: 137-148.
- Bastide NM, Pierre FH, Corpet DE (2011) Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) 4: 177-184.
- Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G (2010) Heme degradation and vascular injury. Antioxid Redox Signal 12: 233-248.
- Huang M, Narita S, Numakura K, Tsuruta H, Saito M, et al. (2012) A high fat diet enhances proliferation of prostate cancer cells and activates MCP-1/ CCR2 signaling. Prostate 72: 1779-1788.
- Chen D, Zhao H, Coon JS, Ono M, Pearson EK, et al. (2012) Weight gain increases human aromatase expression in mammary gland. Mol Cell Endocrinol 355: 114-120.
- Lu YP, Lou YR, Bernard JJ, Peng QY, Li T, et al. (2012) Surgical removal of the parametrial fat pads stimulates apoptosis and inhibits UVB-induced carcinogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 109: 9065-9070.
- Christou NV, Lieberman M, Sampalis F, Sampalis JS (2008) Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis 4: 691-695.
- Helferich WG, Andrade JE, Hoagland MS (2008) Phytoestrogens and breast cancer: a complex story. Inflammopharmacology 16: 219-226.
- Singh SV, Singh K (2012) Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis 33: 1833-1842.
- Tosetti F, Noonan DM, Albini A (2009) Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer 125: 1997-2003.
- Steiner C, Arnould S, Scalbert A, Manach C (2008) Isoflavones and the prevention of breast and prostate cancer: new perspectives opened by nutrigenomics. Br J Nutr 1: ES78-ES108.
- Escrich E, Solanas M, Moral R, Escrich R (2011) Modulatory effects and molecular mechanisms of olive oil and other dietary lipids in breast cancer. Curr Pharm Des 17: 813-830.
- Licciardi PV, Kwa FA, Ververis K, Di Costanzo N, Balcerczyk A, et al. (2012) Influence of natural and synthetic histone deacetylase inhibitors on chromatin. Antioxid Redox Signal 17: 340-354.
- Kang MS, Hirai S, Goto T, Kuroyanagi K, Lee JY, et al. (2008) Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem Biophys Res Commun 369: 333-338.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi