Research Article, J Nanomater Mol Nanotechnol Vol: 7 Issue: 3
A New Insight for Synthesizing Polymeric Nano-Microspheres with Different Size by Using Different Initiators in the Conventional Emulsifier-Free Emulsion Polymerization
Chen S, Chen C, Jiang L* and Dan Y*
State Key Laboratory of Polymer Materials Engineering of China, Sichuan University, Chengdu, China
*Corresponding Authors : Yin Dan
State Key Laboratory of Polymer Materials Engineering of China, Sichuan University, Chengdu, China
E-mail: danyi@scu.edu.cn
Long Jiang
State Key Laboratory of Polymer Materials Engineering of China, Sichuan University, Chengdu, China
E-mail: jianglong@scu.edu.cn
Received: July 03, 2018 Accepted: July 18, 2018 Published: July 23, 2018
Citation: Jiang L, Chen S, Chen C, Dan Y (2018) A New Insight for Synthesizing Polymeric Nano-Microspheres with Different Size by Using Different Initiators in the Conventional Emulsifier-Free Emulsion Polymerization. J Nanomater Mol Nanotechnol 7:3. doi: 10.4172/2324-8777.1000251
Abstract
Using different initiators, potassium persulphate (KPS) and azodiisobutyronitrile (AIBN), two conventional emulsifier-free emulsion polymerizations of the monomer tert-butyl acrylate (t-BA) in aqueous medium were carried out. The results of the measurements on the transmittance of the reaction system and the conversion of the monomer through UV-Vis absorption spectroscopy indicate that the polymerization processes of the t-BA initiated by KPS shows three stages while that initiated by AIBN behaves two stages. Investigations on the morphology and the average hydrodynamic diameter and its distribution of the obtained poly (t-BA) nano-microspheres by scanning electron microscope and dynamic light scattering techniques show that, during the polymerization, the poly(t-BA) nano-microspheres from AIBN initiated polymerization show a larger average hydrodynamic diameter and a steady diameter’s distribution. The results have prompted us to have a new insight in emulsifier-free emulsion polymerization and provide us a new idea and a convenient approach to synthesize polymeric nano-microspheres with a different hydrodynamic diameter by using initiator with required solubility in aqueous medium through free-radical polymerization.
Keywords: Polymeric nano-microsphere; Hydrodynamic diameter and its distribution; Initiator; Solubility; Emulsifier-free emulsion polymerization
Introduction
Polymeric nano-microspheres with high purity are of great importance in such areas as flooding water for the polymer microsphere is a viscoelastic plugging agent with 3-D structure [1], removing dissolved and colloidal substances and inorganic salts in water to ensure paper making [2], drug delivery [3], chromatography medium for protein separation [4], and supporting catalyst etc. and, when the chemical structure is certain, the sphere’s size and size distribution are key issue for the applications. Usually, the polymeric nano-microspheres with high purity are synthesized through emulsifier-free emulsion polymerization a convenient and environmentally friendly technique of the monomer in aqueous medium using water-soluble initiator such as potassium persulphate (KPS) and ammonium persulfate (APS). By this technique, in order to obtain polymeric nano-microspheres with different size, monomer/water ratio, monomer/initiator ratio, reaction temperature and time, and stirring speed are often concerned and investigated, and, in order to get nano-microspheres with larger scale and narrower size distribution, the particle coagulation process is now undertaken [5]. Recently, applications of oil-soluble initiator and water-soluble initiator simultaneously in emulsifier-free emulsion polymerization of styrene in an aqueous medium to get polystyrene nano-microspheres with larger size have been reported [6], but the concentration of styrene is only 64 mmol/L. In our previous research, it was found that, in the emulsifier-free emulsion polymerization of tert-butyl acrylate (t-BA), both water-soluble initiator (e.g., KPS) and oil-soluble initiator (e.g., azodiisobutyronitrile AIBN) could result in formation of poly(tert-butyl acrylate) (poly(t-BA)) nano- microspheres with different hydrodynamic diameter but regular morphology even at higher monomer concentration of 138 mmol/L. This new interesting result prompts and encourages us to have a new insight for synthesizing polymeric nano-microspheres through the convenient and environment-friendly emulsifier-free emulsion polymerization. To understand the result more clearly, in this article, the differences between the two polymerization process and then the hydrodynamic diameter and its distribution of the obtained polymeric nano-microspheres by using different initiators with different solubility will be discussed. The tert-butyl acrylate (t-BA) will be used again as the model monomer because of (1) its characteristics in the solubility in water and oil coming from the ester group in the molecules [7,8] and (2) the applications of the polymer poly(t-BA) in the fields of pH environmental responses [9,10].
Experimental Methods
Materials
Tert-butyl acrylate (t-BA) (97%, Alfa Aesar) was used after being purified through distillation. Azodiisobutyronitrile (AIBN) (Chengdu Kelong Chemical Reagent Factory, China) was used with further purification through recrystallization. Potassium persulphate (KPS, A.P), N,N-methylene bisacrylamide (MBA) and sulfuric acid (H2SO4) and ethanol (C2H5OH) (Chengdu Kelong Chemical Reagent Factory, China) all were used as received. Water used in the experiment was deionized with a purification system (K ≤ 0.08 μs/cm, Chengdu Pincheng Scientific Instruments Company, China).
Emulsifier-free emulsion polymerization of t-BA
Two conventional emulsifier-free emulsion polymerizations of t-BA were performed in a 250 mL four-neck reactor equipped with condenser and mechanical stirrer in aqueous medium at 80ºC using KPS and AIBN as initiators respectively. A typical reaction is as follows. First, the reaction medium (H2O, 200 g), the monomers (t-BA, 6.9∼27.6 mmol and MBA, 0.0013 mmol) were charged in the reactor. Then, the reactor was purged with nitrogen to remove oxygen from the mixture and finally heated to 80ºC. After adding 0.06 mmol of the initiator, the polymerization was started. Four hours later, the polymerization was finished. During the polymerization, interval sampling was operated for monitoring the variation of the transmittance of the reaction system, the conversion of the monomer and the size and size distribution of the polymeric nanomicrospheres. Formulations and results of the polymerization are listed in Table 1, while the chemical structures of the monomers as well as their copolymerization are presented in Scheme 1.
| System | t-BA/mmol | MBA/mmol | KPS/mmol | AIBN/mmol | H2O/g | Result |
|---|---|---|---|---|---|---|
| KPS-1 | 6.9 | 0.0013 | 0.06 | 0 | 200 | No reaction |
| KPS-2 | 13.8 | 0.0013 | 0.06 | 0 | 200 | Stable |
| KPS-3 | 20.7 | 0.0013 | 0.06 | 0 | 200 | Stable |
| KPS-4 | 27.6 | 0.0013 | 0.06 | 0 | 200 | Stable |
| AIBN-1 | 6.9 | 0.0013 | 0 | 0.06 | 200 | No reaction |
| AIBN-2 | 13.8 | 0.0013 | 0 | 0.06 | 200 | Stable |
| AIBN-3 | 20.7 | 0.0013 | 0 | 0.06 | 200 | Stable |
| AIBN-4 | 27.6 | 0.0013 | 0 | 0.06 | 200 | Stable |
Table 1: Formulations and results of the polymerizationa.
Characterization
Transmittance of the reaction system: The transmittance of the reaction system at the different reaction time was measured under 625 nm where the transmittance of the system changed the most obvious by UV-Vis spectrophotometer (UV2300, Shanghai Tianmei Scientific Instruments Company, Shanghai, China).
Conversion of the monomer: The conversion of the monomer t-BA during the polymerization was obtained by measuring the absorbance of the reaction system at 205 nm with a UVVis spectrophotometer (UV2300, Shanghai Tianmei Scientific Instruments Company, Shanghai, China). In the UV-Vis absorb spectrum of monomer t-BA, as shown in Figure 1, three absorb bands ï¼�?260 nm, 235 nm and 205 nm) come from the n-π* transition, π-π* transition and n-σ* transition respectively. Taking absorb at 205 nm as a representative, the absorbance decreases with the polymerization since the polymerization transfers the monomer t-BA to the polymer poly(t-BA), resulting in a reduction of the monomer and then the chromophore concentration. Basing on the absorbance of the reaction system at 205 nm, the concentration of the monomer t-BA can be obtained according to the relationship between the absorbance and the concentration as shown in the inert in Figure 1.
After getting the concentration, the conversion (Xt) of the monomer t-BA was calculated according to the following equation (1), where C0 was the initial concentration of the t-BA, while Ct was the concentration of the t-BA at the reaction time t.
Xt= [(C0-Ct)/C0] × 100% (1)
Molecular weight and its poly index of the poly(t-BA): The molecular weight and its poly index of the poly(t-BA) were analyzed by Gel permeation chromatography (GPC) equipped with an Agilent PL gel column over 350C using THF (Fisher, HPLC grade) as eluent at a flow rate of 1 ml/min. The number (Mn) and weight (Mw) average molecular weights were determined with polystyrene (PSt) standards purchased from Agilent.
Morphology and hydrodynamic diameter and its distribution of the poly(t-BA) nano-microspheres: The morphology of the poly(t-BA) nano-microspheres was observed by scanning electron microscopy (SEM, JEOL JSM-5900LV, Japan), while the hydrodynamic diameter and its distribution were detected by dynamic light scattering (DLS, BI-200 SM goniometer, BI-9000 AT digital correlator, Brookhaven Instruments) at 90°of the testing angle under 532 nm at 25ºC. Through the DLS measurements, the average hydrodynamic diameter (Í�?Dh) and the distribution of the hydrodynamic diameter (Dh) were obtained.
Results and Discussion
Variation of transmittance of the reaction system during the polymerization
The Figure 2 shows the variation of transmittance of the reaction system with the reaction time. It can be seen that systems KPS-2, KPS-3, KPS-4, AIBN-2, and AIBN-3 behave basically the same changing trends, i.e., the transmittance drops sharply in the first 10 min, then decreases slowly, and becomes stable after about 20 min. The reduction of the transmittance indicates a formation of the polymer poly(t-BA) because of its insoluble in the aqueous medium, while the stable transmittance implies a finish of the polymerization of the monomers. Differences between the transmittance of the two initiator systems in the stable state indicate a different contents of the formed poly(t-BA) and hence a different conversion of the monomers. Transmittances of the reaction system using KPS as initiator are lower than that of the reaction system using AIBN as initiator, indicating that the polymerizing rate (as analyzed later on the conversion of the monomers) (Figure 3) and then the forming rate of the poly(t-BA) nano-microspheres (as analyzed later on the Dh of the systems) (Figure 4) of the former (KPS system) are higher than that of the later(AIBN system), When KPS (as shown in Scheme 2a) was used as initiator, the end of the polymer chains usually has some ionic groups (-SO42-) distributing on the surface of the formed poly(t-BA) nano-microspheres. Electrostatic repulsion among the ionic groups could avoid the combination of the spheres and then improve the stability of the reaction system, resulting in the formation of more nano-microspheres. The more nano-microspheres bring stronger refraction and reflection and finally lead to lower transmittance of the system. When AIBN (as shown in Scheme 2b) was used as initiator, however, no any ionic groups exists in the system, smaller spheres would combine together to form a larger spheres (which will be confirmed by the following analysis on the average hydrodynamic diameter and the distribution of the hydrodynamic diameter of the nano-microspheres) due to the stronger interaction between the spheres and then isolate from the aqueous phase, making the detected transmittance being higher. Increasing in the dosage of t-BA monomer, when using AIBN as initiator, results in a non-steady process as shown in the transmittance curve of AIBN-4 system in Figure 2b.The following discusses will focus on the AIBN-2, AIBN-3 and KPS-2, KPS-3.
Variation of conversion of the monomer during the polymerization process
Figure 3 shows and compares variation of conversion of the monomer with the polymerization process under different initiator conditions. Using KPS as initiator, two curves (Figure 3a) show the same trends though the dosage of the monomer is different, having three stages, i.e., the fast increase in conversion in 20 min (area I in Figure 3a), then the reduced increase in conversion between 20 min and about 75 min (area II in Figure 3a), at last almost the constant conversion value after 75 min (area III in Figure 3a). When AIBN was used as the initiator (Figure 3b), however, higher dosage (system AIBN-3) of the monomer leads to faster reaction process in the first 50 min, and conversion of the monomer shows two stages, i.e., the faster increase in conversion in a certain time t, then almost the constant conversion value after the t, and the higher monomer dosage results in a shorter t. The considerable differences in the polymerization kinetics should be from the different solubility of the initiators in the aqueous medium (KPS is water soluble while AIBN is water insoluble) and the different primary free radical generation’s rate (activation energy of KPS is 33.5 kJ/mol, while that of AIBN is 129 kJ/mol [11]). In the emulsifier-free emulsion polymerization of the t-BA monomer, the water-soluble initiator KPS results in a polymerization in the micelles, while the oil-soluble initiator AIBN results in a polymerization in the monomer droplets, following the former researches as reported by Capek [12], Horak [13], Tanrisever et al. [14], Kuhn and Tauer [15], Pavol [16], and Shouldice et al. [17].
Hydrodynamic diameter and its distribution of the poly (t- BA) nano-microspheres
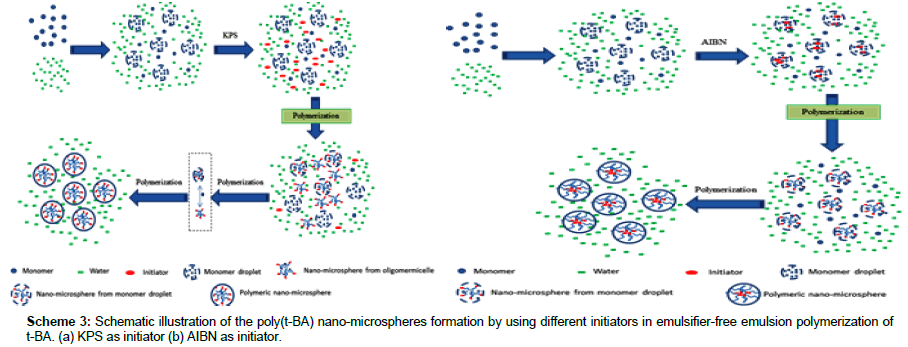
Figure 4 shows that the average hydrodynamic diameter (Dh) of the obtained polymeric nano-microspheres increases gradually in the earlier period and then tend to be stable after a certain timeτ. When AIBN was used as initiator the τ is about 40 min, while that is about 25 min when KPS was used as initiator. By comparing the Figure 4a with Figure 4b, it is obvious that the poly (t-BA) nanomicrospheres obtained by the AIBN initiator (Figure 4b) are much bigger than that obtained by the KPS initiator (Figure 4a). The both results of the variation process and the value of Í�?Dh of the poly (t-BA) nano-microspheres further indicate a different form of the polymeric nano-microspheres when using different initiators. From the images shown in following figures, it can be seen that the polymeric nanomicrospheres obtained by AIBN are indeed much larger than that obtained by KPS (Figure 5). By further carefully observing the SEM images of the two polymeric nano-microspheres, it is found that nanomicrospheres from AIBN initiated polymerization are less uniform and mono disperse than from KPS initiated polymerization, which is also proved by the following measurement on the distribution of hydrodynamic diameter as shown in the following Figure 6a KPS-92 min and Figure 6b AIBN-92 min.
Taking KPS-3(with Mn of 12.70 × 104 g/mol and Mw/Mn of 3.77 measured by the GPC) and AIBN-3 (with Mn of 16.89 × 104 g/mol and Mw/Mn of 3.17 also measured by the GPC) as examples, Distributions of the hydrodynamic diameter of the poly(t-BA) nano-microspheres obtained at 5 min, 35 min and 92 min respectively, were further investigated and results are shown in Figure 6. From Figure 6, it can be clearly seen that different initiators result in different change process of the diameter’s distributions. The distribution of the hydrodynamic diameter of the KPS-initiated system (Figure 6a) changes from a narrower distribution in the very beginning of the polymerization (e.g., in 5 min of the polymerization) to a wider distribution in the early periods of the polymerization (e.g., in 35 min of the polymerization) then to an almost mono-distribution in the later periods of the polymerization (e.g., in 92 min of the polymerization). The distribution of the hydrodynamic diameter of the AIBN-initiated system (Figure 6b), on the other hand, changes little in the whole duration of the polymerization and is of almost steady distribution though the average hydrodynamic diameter of the polymeric nanomicrospheres increases with reaction time. The difference in the change process of the distribution of hydrodynamic diameter implies a different process of the polymeric nano-microsphere’s formation. When KPS was used as initiator (as shown in Scheme 3a), the dissolved initiator molecules (I) in water will decompose to free radicals (R•) in aqueous phase and then initiate the monomer molecules (M) dissolved in aqueous phase (solubility of the t-BA in water is 2 g/L) to form chain radicals (MxR•)ï¼�?then to form oligomer micelles. At very beginning, polymerization occurs mainly in the oligomer micelles, leading to a narrower size distribution of the formed polymeric nanomicrospheres (as shown in Figure 6a, at 5 min, the polymeric nanomicrospheres distributed from about 60 nm to about 80 nm.). As the reaction progresses, the chain radicals (MxR•) diffuses into monomer droplets, the polymerization occurs not only in the oligomer micelles but also in the monomer droplets, resulting in a wider distribution in the hydrodynamic diameter (as shown in Figure 6a, at 35 min, the polymeric nano-microspheres distributed from about 110 nm to about 200 nm). Further polymerization makes a polymeric nanomicrospheres formed in different sites combine together because of the thermodynamics and the dynamics of the reaction, leading to an uniform distribution of the polymeric nano-microspheres (at 92 min, the polymeric nano-microspheres distributed from about 145 nm to about 152 nm). When AIBN was used as initiator (as shown in Scheme 3b), on the other hand, the polymerization occurs only in the monomer droplets because the AIBN is insoluble in water. The mono-nucleation site results in a narrower distribution of the formed polymeric nano-microspheres during the polymerization, and the average hydrodynamic diameter increases with the polymerization, behaving as (as shown in Figure 6b) that the nano-microspheres distributed from about 170 nm to about 180 nm at 5 min, from about 210 nm to about 220 nm at 35 min and from about 226 nm to about 236 nm at 92 min. The bigger nano-microspheres obtained by using AIBN as initiator should be attributed to the nuclear formation in the monomer droplets. These results give us a new insight for synthesizing larger polymeric nano-microspheres by not only convenient but also environment friendly free radical polymerization techniques in aqueous medium.
Conclusions
The free radical polymerizations of a model monomer t-BA initiated with KPS and AIBN respectively in aqueous medium (emulsifier-free emulsion polymerization) were investigated. For a certain monomer, different initiators with different soluble properties induce different nucleation and then different formation of the polymeric nano-microspheres. The polymerization initiated with water-soluble initiator KPS involves oligomer micelle nucleation and monomer droplet nucleation, leading to a variable distribution of hydrodynamic diameter in various periods of the polymerization, while the average hydrodynamic diameter of the final polymeric nano-microsphere is smaller. When AIBN, an oil-soluble initiator, is used to initiate the polymerization, the nucleation occurs only in monomer droplets, resulting in a narrower distribution of hydrodynamic diameter in the whole periods of the polymerization, while the average hydrodynamic diameter of the final polymeric nano-microsphere is larger. This prompts us to have a new insight in emulsifier-free emulsion polymerization and provides us a new idea and a convenient approach to synthesize polymeric nanomicrospheres with a different hydrodynamic diameter by using initiator with required solubility in aqueous medium through freeradical polymerization.
References
- Yang H, Kang W, Yu Y, Yin X, Wang P, et al. (2017) A new approach to evaluate the particle growth and sedimentation of dispersed polymer microsphere profile control system based on multiple light scattering. Powder Technol 315: 477-485.
- Xiao H, He B, Li J, Chen L, Huang L (2017) High performance of hydrophilic polymers on the crosslinked polystyrene spheres for controlling contaminants in white water, J Appl Polym Sci 134: 45169.
- Cheng N, Wang Y, Wu F (2016) Facile fabrication of double-walled polymeric hollow spheres with independent temperature and pH dual-responsiveness for synergetic drug delivery. J Appl Polym Sci 133: 44335.
- Wu R, Xi G, Wang Z, Su Y, Dai L (2018) Preparing polymeric microsphere used in chromatography medium for protein separation, involves using poly(ethylene glycol) methacrylate, methyl methacrylate or styrene as monomer to prepare mono-dispersion micro-sphere.
- Liu B, Sun S, Zhang M, Ren L, Zhang K (2015) Facile synthesis of large scale and narrow particle size distribution polymer particles via control particle coagulation during one-step emulsion polymerization. Colloid Surface A 484: 81-88.
- Yamamoto T, Kawaguchi K, Takahashi Y (2016) Particle size control in the soap-free emulsion polymerization of styrene by an oil-soluble initiator with a weakly acidic water-soluble initiator. Colloid Surface A 502: 1-5.
- Wang Lu, Pan M, Song S, Zhu L, Yuan J, et al. (2016) Intriguing morphology evolution from noncross linked poly(tert-butyl acrylate) seeds with polar functional groups in soap-free emulsion polymerization of styrene. Langmuir 32: 7829-7840.
- Zhao H, Wang B, Li Q, Wang L, Sun J, et al. (2017) Dispersion and soap-free emulsion polymerization of tert-butyl acrylate with the living character and controlled particle size mediated by cobalt porphyrin. RSC Adv 7: 10124-10131.
- Schneider Y, Lynd NA, Kramer EJ, Bazan GC (2009) Novel elastomers prepared by grafting n-butyl acrylate from polyethylene macroinitiator copolymer. Macromolecules 42: 8763-8768.
- Salari JWO, Heck JV, Klumperman B (2010) Steric stabilization of pickering emulsions for the efficient synthesis of polymeric microcapsules. Langmuir 26: 14929-14936.
- Wang H, Kou X (2007) Course for polymer chemistry. (2nd edtn) Science Press of China, Beijing, China
- Capek I (2001) On the role of oil-soluble initiators in the radical polymerization of micellar systems, Adv Colloid Interfac 91: 295-334.
- Horak D (1996) Uniform polymer beads of micrometer size. Acta Polym 47: 20-28.
- Tanrisever T, Okay O, Sonmezoglu IC (1996) Kinetics of emulsifier-free emulsion polymerization of methyl methacrylate. J Appl Polym Sci 61: 485-493.
- Kuhn I, Tauer K (1995) Nucleation in emulsion polymerization: A new experimental study. 1. Surfactant-free emulsion polymerization of styrene. Macromolecules 28: 8122-8128.
- Pavol P (1995) Microemulsion and emulsion polymerization of butyl acrylate-I. Effect of the initiator type and temperature. Eur Polym J 31: 1269-1277.
- Shouldice GTD, Vandezande GA, Rudin A (1994) Practical aspects of the emulsifier-free emulsion polymerization of styrene. Eur Polym J 30: 179-183.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi