Case Report, J Forensic Toxicol Pharmacol Vol: 7 Issue: 1
A Case Report on the Analysis of Poisonous Alkaloids in Delphinium Plant by Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry
Sang Hwan In, Sanggil Choe*, Suncheun Kim, Young Joon Jeon and IlUng Seol
National Forensic Service, Wonju, Republic of Korea
*Corresponding Author : S Choe
National Forensic Service, Seoul institute, 139 Jiyang-ro, Seoul 08036, Republic of Korea
Tel: 82-2-2600-4920
Fax: 82-2-2600-4929
E-mail: csk91@korea.kr
Received: February 01, 2018 Accepted: February 20, 2018 Published: February 26, 2018
Citation: In SH, Choi S, Jeon YJ, Seol I (2018) A Case Report on the Analysis of Poisonous Alkaloids in Delphinium Plant by Liquid Chromatography- Quadrupole Time-of-Flight Mass Spectrometry. J Forensic Toxicol Pharmacol 7:1. doi: 10.4172/2325-9841.1000159
Abstract
A 62-year-old male transferred to a hospital emergency room mentioned that he had drunk a liquor extract from ‘Pokeberry’ (Phytolacca americana). The liquor was summited, dehydrobrownine and delphatine, which were found in plants of the genus Delphinium, were detected via gas chromatography-mass spectrometry (GCMS). High-resolution quadrupole time-of-flight/mass spectrometry (QTOF/MS) can be used for toxicant identification without reference materials, and the exact mass of the target compound and MSMS fragmentation and isotope ratios can be obtained. Based on this integrated information, a comparison of the chemical structure and fragment ions can be used to identify target compounds. From liquid chromatography-QTOF/MS (LC-QTOF/MS) analysis, delsoline, delphinine, 14-acetylbrowniine, dihydrogadesine, and neoline were identified. These diterpenoid alkaloids are wellknown for intoxicating herbivorous livestock in North America, but this was the first intoxication case by genus Delphinium plants in South Korea. Through this study, LC-QTOF/MS was proven as an effective method for addressing intoxication cases in the absence of reference materials. The experience, knowledge, and analytical methods obtained in this study are a great asset for solving other potential natural phytotoxin intoxication cases.
Keywords: Delphinium; GC-MS; LC-QTOF/MS; Toxicant identification; Absence of reference materials; Natural phytotoxin
Introduction
Plants in the Ranunculaceae family and Delphinium genus, which are commonly known as larkspurs in Korea, are widely distributed mostly in the Northern Hemisphere, including Asia, Europe, and North America. Worldwide, there are approximately 350 known species, with 173 species found in China [1]. Plants from the genus Delphinium have been used for a long time for maintaining health, while Delphinium denudatum wall has been used for migraines, epilepsy, mania, paralysis, rheumatism, toothache, and various types of pain [2,3]. Their roots are termed as “Jadwar,” which has been reported to provide anticonvulsant properties that have been traditionally used in Pakistan [4].
Extensive studies have been performed analyzing the components of numerous species in the Delphinium genus, and various types of C18- and C19-diterpenoids and C20-diterpenes have been discovered. Methyllycaconitine is one of the most widely known alkaloids, and its analogues have been reported to provide selective antagonistic action against neuronal α7 nAChR, which is a target of drug development for treating Alzheimer’s disease [5]. Moreover, methyllycaconitine and its analogues block neuromuscular transmission in the musculoskeletal system, causing patients to show symptoms of intoxication similar to d-tubocurarine. In North America, consumption of these compounds has been reported to be the cause of 2–5% of cow deaths [6]. Poisoning from Delphinium barbeyi Huth, which is commonly called “tall larkspur,” causes severe stomach bloating, as well as vascular congestion of the skin and severe kidney congestion, leading to death by respiratory paralysis, while autopsy shows inflamed stomachs, intestines, and respiratory tracts [7].
Toxic alkaloids contained in Delphinium can be divided into three groups depending on their structure and action: N-(methylsuccinyl) anthranoyllycoctonine (MSAL), which possesses the highest toxicity; lycoctonines with medium toxicity; and 7,8-methylenedioxylycoctonine (MDL) with the lowest toxicity. Approximately 40 types of toxic alkaloids have been reported, including methyllycaconitine, delphinine, delcosine, delsoline, lycoctonine, brownine, condelphine, and delphatine (Figure 1) [8-11].
Among Delphinium plants, larkspur (Delphinium grandiflorum L.), which is found throughout the Korea, is a perennial grass that grows in high-altitude grasslands. Its stem stands upright with branches, and it grows to a height of 30-60 cm with short, soft bent hairs. It’s long, split palmate leaves alternate, while its flowers bloom during July and August and forms light-blue racemes at the tip of the stem. The fruit is an aggregate or follicle, while its stems and flowers have been used as ingredients in drugs for treating gastrointestinal diseases, convulsions, and paralysis.
Plants that belong to the same family of larkspurs found in Korea include the Siberian larkspur (D. ornatum Bouche) and Maack’s larkspur (D. maackianum Regel) originating from southern Europe; Bujeon larkspur (D. maackianum var. lasiophyllum), that have hairless fruits but hairy leaves; hairy Maack’s larkspur (D. maackianum Regel var. lasiocarpum Nakai) with hairy fruits and body; and white Maack’s larkspur (D. maackianum for. album) with white flowers. Among these, Maack’s larkspur is primarily found in Korea (Figure 2) [12].
To analyze, the diterpenoid alkaloids contained in Delphinium genus plant, components in the plant body have been analyzed using GC-MS [13,14], while deltaline, delphatine, and methyllycaconitine from cow and goat plasma have been analyzed using positive electrospray mass spectrometry [15,16]. However, new methods are needed for identifying an intoxicant in cases where a reference material is unavailable, and recently the screening and identification by LC-QTOF/MS has been proposed to be an effective method.
This study involved a case of a patient who was transported to the emergency room after intoxication from drinking a pokeberry liquor extract, and toxicants remaining in the liquor were identified using LC-QTOF/MS. Accordingly, this study suggests the need for developing a new testing method by verifying the utility of LC-QTOF/ MS, which has received recent attention for identifying compounds in cases involving intoxication from natural toxicants with no reference materials available.
Case Report
Sometime in September 2016, a 62-year-old male was transferred to the emergency room at Chungbuk National University Hospital after collapsing following ingestion of a homemade liquor extract. The patient stated that he drank pokeberry extract, and to test the intoxicant, approximately 200 mL of the extract and two mug cups were tested.
Methods
Pokeberry (Phytolacca esculenta V. Houtte, Phytolaccaceae family), which the patient had stated that he drank, is a known poisonous plant that contains phytolaccatoxins that cause central nervous system paralysis, respiratory impairment, and blood pressure fluctuation. In Korea, American pokeweed (P. americana L.), a naturalized species, is mostly found in the wild. Therefore, there was a high probability that the pokeberry that the patient had consumed was American pokeweed. Because the components in this plant are glycoside compounds with large molecular weights, analysis using GC-MS, which is used primarily for drug toxicity screening, was difficult, and since the standard materials for these toxicants were unavailable, they could not be analyzed by LC-MS.
After pretreating the sample, GC-MS was performed first to detect and accurately identify other plant-derived components other than the toxicants. GC-MS detected delphatine, a component found in Delphinium, and as a result, it was determined that intoxication was caused by it, not pokeberry. Consequently, the present study analyzed diterpenoid alkaloids that are toxicants found in those plants.
Toxicants found in Delphinium are also difficult to analyze by GCMS. Their material spectrum is not included in commercial libraries, and a standard material for these toxicants is also unavailable. Therefore, the present study used LC-QTOP/MS, which can identify chemical with standard materials by their exact mass, isotope pattern and ratio, and MS/MS, to identify the toxicants.
Sample preparation
The sample was an ethanol containing extract, which made direct extraction via an organic solvent impossible. Therefore, a nitrogen evaporator was used to volatilize the ethanol component first, after which the remaining water layer was used. After making the water layer alkaline by adding 1N-NaOH, 3 mL of ethyl acetate was added over 2 rounds of extraction. The extracts from each round were combined and extracted with nitrogen for analysis by GC-MS and LC-QTOF/MS.
Instrumental analysis conditions
GC-MS analysis conditions: For GC-MS, a 5975C mass selective detector (MSD) and HP7890 GC equipped with a HP7673 (Agilent Technologies, Santa Clara, CA, USA) were used. The column used was a HP-5MS (0.25mm x 0.25μm x 30m). The oven initial temperature was set to 50°C and maintained for 2min, after which the temperature was increased at a rate of 10°C/min up to 280°C and maintained for 10min. The temperatures for the injector and transfer lines were 250°C and 280°C, respectively, while the mobile phase was maintained at 1mL/min with helium gas.
Ionization was performed at 70eV in the electron ionization (EI) mode, while the measurement range was 40-550 amu, and 1 μL was injected by splitless mode.
LC-QTOF/MS analysis conditions: The pretreated sample was analyzed by LC-QTOF/MS. A Nanospace SI-2 LC system (Shiseido, Tokyo, Japan) was used, and the column was a Kinetex 2.6 μ F5 100A (100×2.1 mm), while the oven temperature was set to 40°C. The solvents used were 0.1% acetic acid in deionized water (A) and 0.1% acetic acid in MeOH (B), and analysis was performed using gradients with the conditions described below. The total analysis time was 24min (Table 1).
| Time (min) | Flow rate (μL/min) | B% | A% |
|---|---|---|---|
| Initial | 300 | 5.0 | 95.0 |
| 10.0 | 300 | 5.0 | 95.0 |
| 10.5 | 300 | 5.0 | 95.0 |
| 20.0 | 300 | 95.0 | 5.0 |
| 22.0 | 300 | 95.0 | 5.0 |
| 23.0 | 300 | 5.0 | 95.0 |
| 24.0 | 300 | 5.0 | 95.0 |
Table 1: Gradient condition of mobile phase.
The QTOF/MS apparatus was a TripleTOF 6600 Mass (Sciex, Framingham, MA, USA). Ionization was performed in its electronspray (ESI) positive mode. For TOF/MS and product ion scan, the settings were: ion source gas 1: 50; ion source gas 2: 50; curtain gas: 25; and ion spray voltage: 5500 V. The declustering potential and collision energy were 80 and 40 for the TOF/MS scan, respectively, and 80 and 10 for the product ion scan, respectively.
Results
GC-MS analysis results
GC-MS analysis was performed on the sample from the sitedetected dehydrobrownine (m/z 434, 450, 464) at 15.74min and delphatine (m/z 450, 432, 466, 481) at 17.94min. Dehydrobrownine has been detected in D. geyeri and D. glaucescens, while delphatine is an amorphous crystal that is synonymous with 18-O-methyllycoctonine or delsonine, and has been detected aboveground and in the roots and fruits of D. consolida and D. biternatum, and in the fruits of D. ajacis (Figure 3) [17].
Based on the GC-MS analysis, intoxication was caused by a toxicant contained in the Delphinium genus plant and not by pokeberry as the patient had stated. Therefore, LC-QTOF/MS analysis was performed to identify the toxicants reported in Delphinium genus plants.
LC-QTOF/MS analysis results
Toxicants in Delphinium genus plants were first investigated via a literature review. Over 50 components have been reported, but the exact chemical formula and molecular structure of only 42 components is known. Subsequently, a list was created with the species in which these components were found (Table 2).
| No. | Name | Formula | Mono isotopic mass | Species |
|---|---|---|---|---|
| 1 | 14-acetylbrowniine | C27H43NO8 | 509.2989 | D. geyeri D. glaucum |
| 2 | 14-acetyldelcosine | C26H41NO8 | 495.2832 | D. geyeri |
| 3 | 14-acetylvirescenine | C25H39NO7 | 465.2727 | D. virescens |
| 4 | 14-deacetylnudicauline | C36H48N2O10 | 668.3309 | D. pentagynum |
| 5 | 14-dehydrobrowniine | C25H39NO7 | 465.2727 | D. geyeri D. glaucescens Aconitum kirinense |
| 6 | Ajaconine | C22H33NO3 | 359.2460 | D. ajacis D. virescens |
| 7 | Anthranoyllycoctonine | C32H46N2O8 | 586.3254 | D. barbeyi D. glaucescens |
| 8 | Bicoloridine | C25H39NO6 | 449.2777 | D. bicolor |
| 9 | Bicolorine | C22H35NO5 | 393.2515 | D. bicolor |
| 10 | Browniine | C25H41NO7 | 467.2883 | D. brownii D. cardinale D. geyeri D. glaucescens D. glaucum D. biternatum D. virescens |
| 11 | Chasmanthinine | C36H49NO9 | 639.3407 | D. confusum Aconitum chasmanthium |
| 12 | Condelphine | C25H39NO6 | 449.2777 | D. bicolor D. denudatum |
| 13 | Delatine(hetisine) | C19H25NO3 | 315.1834 | D. elatum |
| 14 | Delcosine | C24H39NO7 | 453.2727 | D. barbeyi D. bicolor D. geyeri D. glaucescens D. occidentale D. consolida |
| 15 | Delphatine | C26H43NO7 | 481.3040 | D.geyeri D.biternatum D.consolida D.ajacis |
| 16 | Delphelatine | C30H47NO9 | 565.3251 | D. elatum |
| 17 | Delpheline | C25H39NO6 | 449.2777 | D. barbeyi D. occidentale D. elatum |
| 18 | Delphinine | C33H45NO9 | 599.3094 | D. staphisagria |
| 19 | Delphonine | C24H39NO7 | 453.2727 | D. sp. |
| 20 | Delsoline | C25H41NO7 | 467.2883 | D. occidentale D. consolida |
| 21 | Deltaline | C27H41NO8 | 507.2832 | D. barbeyi D. glaucescens D. occidentale D. elatum |
| 22 | Deltamine | C9H8N2O2 | 176.0586 | D. barbeyi D. occidentale |
| 23 | Denudatine | C22H33NO2 | 343.2511 | D. sp. |
| 24 | Dictyocarpine | C26H39NO8 | 493.2676 | D. barbeyi D. geyeri D. glaucescens D. occidentale |
| 25 | Dictyocarpinine | C24H37NO7 | 451.2570 | D. glaucescens |
| 26 | Dihydrogadesine | C23H37NO6 | 423.2621 | D. pentagynum |
| 27 | Gadenine | C30H41NO8 | 543.2832 | D. pentagynum |
| 28 | Gadesine | C23H35NO6 | 421.2464 | D. pentagynum |
| 29 | Geyeridine | C22H27NO5 | 385.1889 | D. geyeri |
| 30 | Geyerine | C25H33NO5 | 427.2359 | D. geyeri |
| 31 | Geyerinine | C27H37NO7 | 487.2570 | D. geyeri |
| 32 | Glaucerine | C30H45NO9 | 563.3094 | D. geyeri D. glaucescens |
| 33 | Karakoline | C22H35NO4 | 377.2566 | D. bicolor D. pentagynum |
| 34 | Lycoctonine(royline) | C25H41NO7 | 467.2883 | D. barbeyi D. bircolor D. glaucescens D. glaucum D. tricorne |
| 35 | Methyllycaconitine | C37H50N2O10 | 682.3466 | D. bicolor D. glaucescens D. glaucum D. nuttallianum D. tricorne D. elatum |
| 36 | Neoline | C24H39NO6 | 437.2777 | D. pentagynum |
| 37 | Nudicauline | C38H50N2O11 | 710.3415 | D. nuttallianum |
| 38 | Pentagynine | C23H35NO5 | 405.2515 | D. pentagynum |
| 39 | Tricornine | C27H43NO8 | 509.2989 | D. tricorne |
| 40 | Glaucenine | C31H47NO9 | 577.3251 | D. geyeri D. glaucescens |
| 41 | Delvestine | C32H46N2O8 | 586.3254 | D. vestitum |
| 42 | Delvestidine | C33H48N2O8 | 600.3411 | D. vestitum |
Table 2: Alkaloids of Delphinium plants.
QTOF/MS can identify substances by accurately measuring their molecular weight, but cannot distinguish positional isomers with the same chemical formula, only those with different chemical structures. As shown in Table 2, numerous compounds share chemical formulas: delcosine and delphonine (C24H39NO7); bicoloridine, condelphine and delpheline (C25H39NO6); 14-acetylvirescenine and 14-dehydrobrownine (C25H39NO7); brownine, delsoline, and lycoctonine (C25H41NO7); 14-acetylbrownine and tricornine (C27H43NO8); and anthranoyllycoctonine and devestine (C32H46N2O8).
To confirm the detection of the 42 target compounds identified from the QTOF/MS analysis data and literature review, the Masterview® software from Sciex was used. The compounds shown in Table 3 were detected by processing and generating the exact mass values of H+, Na+, and NH4+ adduct and formulas of target compounds. Among the compounds detected, delsoline, brownine, and lycoctonine share the chemical formula C25H41NO7; 14-acetylbrownine and tricornine share C27H43NO8; and delcosine and delphonine share C24H39NO7. As a result, a final component determination by QTOF/ MS was not possible. To exactly identify the components, a direct comparison with available standard materials was needed, along with structural identification using isolated and purified components from Delphinium genus plants.
| Name | Formula | Adduct | Mono isotopic mass | Extraction mass | Found at mass | Error (ppm) | RT | Area |
|---|---|---|---|---|---|---|---|---|
| Delsoline Browniine Lycoctonine (royline) |
C25H41NO7 | H+ | 467.2883 | 468.2956 | 468.2942 | 3.0 | 3.69 | 16718 |
| 14-acetylbrowniine tricornine |
C27H43NO8 | H+ | 509.2989 | 510.3061 | 510.3064 | 0.4 | 4.34 | 32186 |
| Delcosine Delphonine |
C24H39NO7 | H+ | 453.2727 | 454.2799 | 454.2802 | 0.6 | 3.08 | 32833 |
| Delphinine | C33H45NO9 | H+ | 599.3094 | 600.3167 | 600.3155 | -2.0 | 4.66 | 39167 |
| Dihydrogadesine | C23H37NO6 | H+ | 423.2621 | 424.2694 | 424.2697 | 0.7 | 2.76 | 18887 |
| Neoline | C24H39NO6 | H+ | 437.2777 | 438.2850 | 438.2858 | 1.8 | 3.39 | 676464 |
Table 3: Toxic diterpenoid alkaloids detected in evidence.
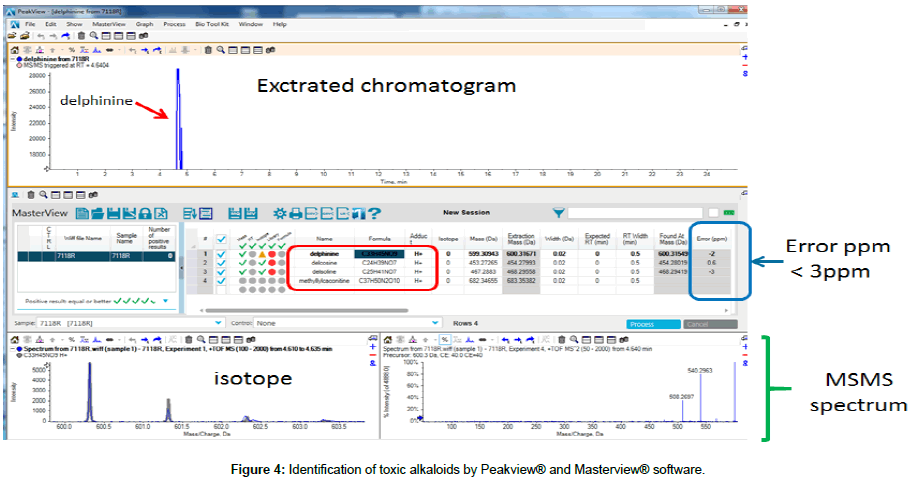
The Peakview® and Masterview® software from Scienx were used to identify the isotope ratios, in addition to the exact mass values of the target compounds. Differences (in %) were derived by comparing the theoretical isotope values based on the chemical formula of the component to the actual measured value of that isotope (Figure 4).
Another method to differentiate materials employs quadrupoles with high-resolution TOF/MS to verify MS/MS data. Energy was again applied to the base ions obtained from TOF/MS to generate fragments, and the product ions obtained were compared with material structures using software to confirm the accurate material matches.
Whether the fragmented ions match the patterns fragmented from the molecular structure must be confirmed by matching them with the component chemical structure obtained via the QTOF/ MSMS spectrum. Since these fragmented ions have exact masses, the material can be identified more accurately by checking the error parts-per-million value (Figure 5).
Discussion
When a standard material is unavailable, high-resolution Q-TOF/ MS can be used to measure the exact mass of a toxicant, and to more accurately identify the material, a MS/MS spectrum can be obtained and compared to the chemical structure. In the present case, a patient was transferred to a university hospital due to intoxication putatively caused by pokeberry extract ingestion. However, GC-MS screening confirmed that intoxication was caused instead by the toxicants dehydrobrownine and delphatine found in plants from the genus Delphinium, not pokeberry, correcting a possible misdiagnosis.
Delphinium genus plants are well-known to be poisonous, and contain components with chemical structures similar to aconitine, a major toxicant found in aconites traditionally used in Korea as ingredients in herbal medicine and poison. In North America, Delphinium is common and frequent cases of livestock, including cows, horses, goats, and sheep occur resulting in death due to poisoning after ingesting these plants. Consequently, many studies have been performed on these plants. However, in Korea, virtually no studies have appeared since Delphinium is not common, and particularly since cases of livestock or human intoxication are rare.
In this study, the patient statement was not accurate, and with no standard material available, GC-MS analysis was performed on the liquor left by the patient. Although dehydrobrownine, delphatine, and structural isomers with identical chemical formulas could not be clearly identified, delsoline, delphinine, 14-acetylbrownine, delcosine, dihydrogadesine, and neoline were identified by LC-QTOF/MS, confirming that the patient was poisoned by toxic alkaloids of the diterpenoid contained in Delphinium-genus plants. This effort confirmed that LC-QTOF/MS can be an effective method for analyzing samples in intoxication cases involving natural toxicants, for which securing standards can be difficult. The experience and knowledge gained, and analysis methods used may be applied as a good sample case for future intoxication cases involving natural toxicants.
Acknowledgement
This work was supported by National Forensic Service (NFS2018-DNT01), Ministry of the Interior, Republic of Korea.
References
- Wang W, Michael JW, Wu ZY, Raven PH, Hong DY (2007) Flora of China Eds. Science Press Beijing 6: 223.
- Pelletier SW, Benn MH, Jacyno JM (1984) The Alkaloids, chemical and biological perspective. J Wiley Sons 153.
- Nizami Q, Jafri MA, Unani drug (2006) Jadwar (Delphinium denudatum Wall.)- A review. Indian Journal of Traditional Knowledge 5: 463-467.
- Yunusov MS (1991) Diterpenoid alkaloids. Nat Prod Rep 8: 499.
- Yum L, Wolf K, Chiappinelli V (1996) Nicotinic acetylcholine receptors in separate brain regions exhibit different affinities for methyllycaconitine. Neuroscience 72: 545-555.
- Ralphs M, Lynn J (2002) Chemotaxonomy, toxicity, and management of three species of tall larkspur. Biochemical Systematics and Ecology 30: 75-76.
- Gamble JA (1929) Cooling milk and cream on the farm. US Department of Agriculture 13.
- Wang FP, Yan LP (2007) Campylopin from Delphinium campylocentrum, the first hetidane C20-diterpene, suggests a new alkaloid biogenetic pathway. Tetrahedron 63: 1417-1420.
- Díaz JG, García R, Werner H (2004) Alkaloids from Delphinium pentagynum. Phytochemistry 65: 2123-2127.
- Pelletier SW, Dailey Jr. OD, Mody NV, Olsen JD (1981) Isolation and structure elucidation of the alkaloids of Delphinium glaucescens Rydb. J Org Chem 46: 3284-3293.
- González AG, de la Fuente G, Díaz R (1982) Four new diterpenoid alkaloids from Delphinium pentagynum. Phytochemistry 21: 1781-1782.
- Lee CB (1985) An Illustrated guide to Korean Flora. (3rd edtn), HY press, Seoul, Republic of Korea.
- Michelle DM, Frank RS (1997) Transfer of alkaloids from delphinium to castilleja via root parasitism. norditerpenoid alkaloid analysis by electrospray mass spectrometry. Biochemical Systematics and Ecology 25: 279-285.
- Manners GD (1989) Capillary gas chromatography od Delphinium deterpenoid alkaloids. J Chromatography 466: 427-432.
- Welch KD, Gardner DR, Stonecipher CA, Green BT, Pfister JA (2017) Serum toxicokinetics after intravenous and oral dosing of larkspur toxins in goats. Toxicon 133: 91-94.
- Welch KD, Stonecipher CA, Green BT, Gardner DR, Cook D, Pfister JA (2017) Administering multiple doses of a non N-(methylsuccinimido) anthranoyllycoctonine (MSAL)-containing tall larkspur (Delphinium occidentale) to cattle. Toxicon 128: 46-49.
- Buckingham J (1997) Dictionary of Natural Products. Suppliment 1. Champman & Hall Chemical Database 1-615.
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi