Citiation Regenerative medicine
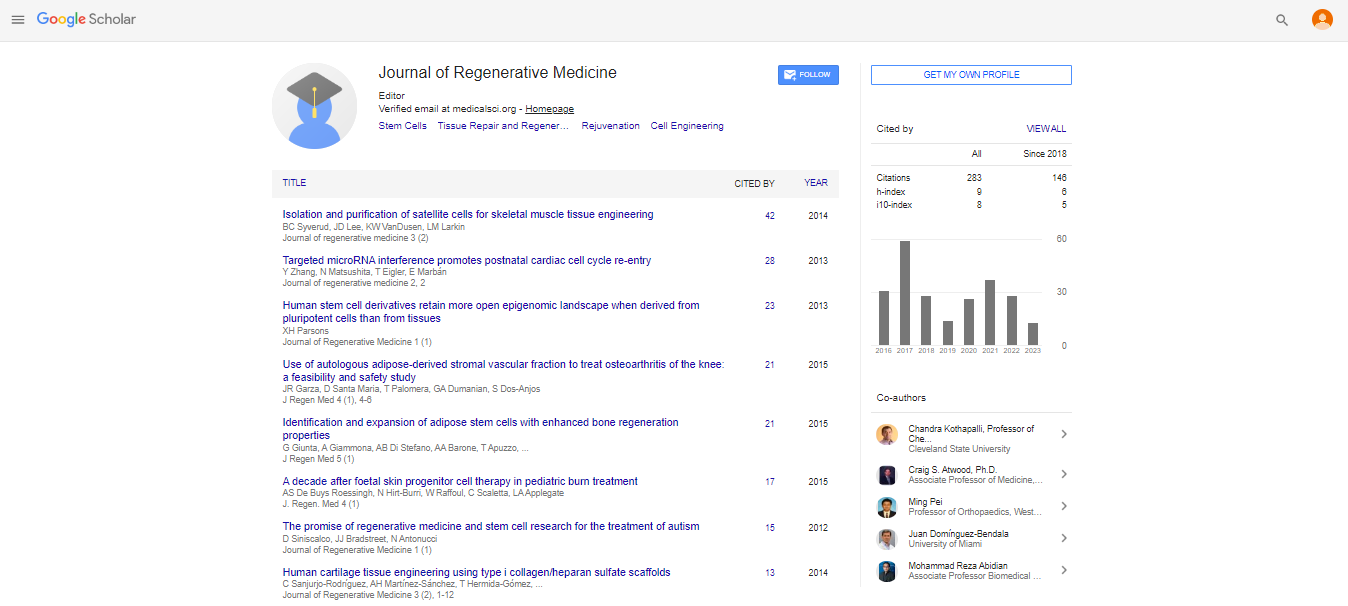
Articles published in Journal of Regenerative Medicine have been cited by esteemed scholars and scientists all around the world. Journal of Regenerative Medicine has got h-index 12, which means every article in Journal of Regenerative Medicine has got 12 average citations.
Following are the list of articles that have cited the articles published in Journal of Regenerative Medicine.
| 2024 | 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2016 | 0 | |
|---|---|---|---|---|---|---|---|---|---|
Total published articles |
61 | 51 | 30 | 31 | 20 | 32 | 5 | 9 | 0 |
Conference proceedings |
0 | 13 | 5 | 0 | 0 | 50 | 187 | 0 | 0 |
Citations received as per Google Scholar, other indexing platforms and portals |
21 | 46 | 39 | 43 | 33 | 14 | 28 | 32 | 64 |
| Journal total citations count | 383 |
| Journal Impact Factor | 5.80 |
| Journal 5 years Impact Factor | 3.04 |
| Journal CiteScore | 6.53 |
| Journal h-index | 12 |
| Journal h-index since 2017 | 15 |
Sanjurjo-RodrÃguez, C., MartÃnez-Sánchez, A. H., Hermida-Gómez, T., Fuentes-Boquete, I., DÃaz-Prado, S., & Blanco, F. J. (2016). Differentiation of human mesenchymal stromal cells cultured on collagen sponges for cartilage repair. Histol Histopathol, 31(11), 1221-1239. |
Sanjurjo-Rodriguez, C., Castro-Vinuelas, R., Hermida-Gomez, T., Fernandez-Vazquez, T., Fuentes-Boquete, I. M., de Toro-Santos, F. J., ... & Blanco-GarcÃa, F. J. (2017). Ovine mesenchymal stromal cells: morphologic, phenotypic and functional characterization for osteochondral tissue engineering. PLoS One, 12(1), e0171231. |
Aronowitz, J. A., Lockhart, R. A., Hakakian, C. S., & Birnbaum, Z. E. (2016). Adipose stromal vascular fraction isolation: A head-to-head comparison of 4 cell separation systems# 2. Annals of plastic surgery, 77(3), 354-362. |
Gonçalves, S. (2016). New therapeutic strategies for osteoarthritis: injective cell therapy (Doctoral dissertation). |
Young, B. K., Chan, M. K., Liu, L., & Basch, R. S. (2016). Amniotic fluid as a source of multipotent cells for clinical use.ÃÂ Journal of perinatal medicine,ÃÂ 44(3), 333-337. |
Dziadosz, M., Basch, R. S., & Young, B. K. (2016). Human amniotic fluid: a source of stem cells for possible therapeutic use.ÃÂ American journal of obstetrics and gynecology,ÃÂ 214(3), 321-327. |
KováÃÂ, M., KulÃÂková, B., Vaà ¡ÃÂÃÂek, J., & Chrenek, P. (2016). Cryopreservation of amniotic fluid stem cells derived from Zobor rabbit.àSlovak Journal of Animal Science,à49(2), 62-67. |
Dziadosz, M., Chan, M., Basch, R., & Young, B. K. (2016). Effects of Pharmacological Agents on Human Amniotic Fluid-Derived Stem Cells in Culture.ÃÂ Stem Cells and Development,ÃÂ 25(20), 1570-1579. |
Ben-Arye, T., & Levenberg, S. (2019). Tissue engineering for clean meat production. Frontiers in Sustainable Food Systems, 3, 46. |
Esmaeli, A., Moshrefi, M., Shamsara, A., Eftekhar-vaghefi, S. H., & Nematollahi-mahani, S. N. (2016). Xeno-free culture condition for human bone marrow and umbilical cord matrix-derived mesenchymal stem/stromal cells using human umbilical cord blood serum.ÃÂ International Journal of Reproductive BioMedicine,ÃÂ 14(9), 567. |
Atkinson, P. J., Nicholson, M., & Richardson, R. T. Progressing towards a cure for deafness through gene therapy. |
Dimitropoulos, G., Jafari, P., de Buys Roessingh, A., Hirt-Burri, N., Raffoul, W., & LA1�õ, A. BURN PATIENT CARE LOST IN GOOD MANUFACTURING PRACTICES?. |
Sharma, A., Gokulchandran, N., Sane, H., Nagrajan, A., Paranjape, A., Kulkarni, P., ... & Badhe, P. (2013). Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem cells international, 2013. |
Bradstreet, J. J., Sych, N., Antonucci, N., Klunnik, M., Ivankova, O., Matyashchuk, I., ... & Siniscalco, D. (2014). Efficacy of fetal stem cell transplantation in autism spectrum disorders: an open-labeled pilot study. Cell Transplantation, 23(1_suppl), 105-112. |
Siniscalco, D., Kannan, S., Semprún-Hernández, N., Eshraghi, A. A., Brigida, A. L., & Antonucci, N. (2018). Stem cell therapy in autism: recent insights. Stem cells and cloning: advances and applications, 11, 55. |
Siniscalco, D., & Antonucci, N. (2013). Possible use of Trichuris suis ova in autism spectrum disorders therapy. Medical Hypotheses, 81(1), 1-4. |
Khongrum, J., & Wattanathorn, J. (2015). Laser acupuncture improves behavioral disorders and brain oxidative stress status in the valproic acid rat model of autism. Journal of Acupuncture and Meridian Studies, 8(4), 183-191. |
Siniscalco, D., & Antonucci, N. (2013). Involvement of dietary bioactive proteins and peptides in autism spectrum disorders. Current Protein and Peptide Science, 14(8), 674-679. |
Sharma, A., Sane, H., Gokulchandran, N., Badhe, P., Patil, A., Kulkarni, P., & Paranjape, A. (2016). PET-CT scan shows decreased severity of autism after autologous cellular therapy: a case report. Autism Open Access, 6(169), 2. |
Siniscalco, D., & Antonucci, N. (2015). Nuclear Magnetic Resonance Spectroscopy in the Diagnosis of Autism-Related Disorders. In Applications of NMR Spectroscopy (pp. 131-142). Bentham Science Publishers. |
Sharma, A. K., Sane, H., Kulkarni, M. P., Gokulchandran, N., & Badhe, P. Cellular therapy in Neurodevelopmental disorders. brain injury, 6, 11. |
Siniscalco, D., & Antonucci, N. (2013). Role of Proteases in Autism Spectrum Disorders. In Proteases in Health and Disease (pp. 327-333). Springer, New York, NY. |
Brigida, A. L., & Siniscalco, D. (2016). Induced pluripotent stem cells as a cellular model for studying Down Syndrome. Journal of stem cells & regenerative medicine, 12(2), 54. |
Farrell, K., Joshi, J., & Kothapalli, C. R. (2017). Injectable uncrosslinked biomimetic hydrogels as candidate scaffolds for neural stem cell delivery. Journal of Biomedical Materials Research Part A, 105(3), 790-805. |
Jalili-Firoozinezhad, S., Mirakhori, F., & Baharvand, H. (2015). Nanotissue Engineering of Neural Cells. Stem Cell Nanoeng, 265, 265-283. |
Gupta, P., & Shetty, H. Research & Reviews: Journal of Pharmaceutics and Nanotechnology. |
Farrell, K. W. (2016). Role of Matrix Microenviroment on Neural Stem Cell Phenotype and Differentiation under Healthy and Inflammatory Conditions (Doctoral dissertation, Cleveland State University). |
Navarro, X., Torres-EspÃn, A., Allodi, I., Santos, D., González-Pérez, F., Udina, E., & del Valle, J. (2016). Analysis of axonal growth in organotypic neural cultures. |
Siniscalco, D., Bradstreet, J. J., Sych, N., & Antonucci, N. (2014). Mesenchymal stem cells in treating autism: Novel insights. World journal of stem cells, 6(2), 173. |
Sych, N., Klunnik, M., Ivankova, O., Matyaschuk, I., Demchuk, M., Novytska, A., ... & Siniscalco, D. (2014). Efficacy of fetal stem cells in Duchenne muscular dystrophy therapy. Journal of Neurorestoratology, 2(1), 37-46. |
Siniscalco, D., & Sych, N. (2015). Stem cell transplantation for nervous system disorders in Italy, European Union, and Ukraine: Clinical approach and governmental policies. Translational Neuroscience and Clinics, 1(2), 125-127. |
Parsons, X. H. (2013). Embedding the future of regenerative medicine into the open epigenomic landscape of pluripotent human embryonic stem cells. Annual research & review in biology, 3(4), 323. |
Parsons, X. H. (2013). Constraining the Pluripotent Fate of Human Embryonic Stem Cells for Tissue Engineering and Cell TherapyâThe Turning Point of Cell-Based Regenerative Medicine. British biotechnology journal, 3(4), 424. |
Broccoli, V., Colasante, G., Sessa, A., & Rubio, A. (2015). Histone modifications controlling native and induced neural stem cell identity. Current opinion in genetics & development, 34, 95-101. |
Parsons, X. H. (2014). Direct conversion of pluripotent human embryonic stem cells under defined culture conditions into human neuronal or cardiomyocyte cell therapy derivatives. In Human Embryonic Stem Cell Protocols (pp. 299-318). Humana Press, New York, NY. |
Ghosh, D., Mehta, N., Patil, A., & Sengupta, J. (2016). Ethical issues in biomedical use of human embryonic stem cells (hESCs). Journal of Reproductive Health and Medicine, 2, S37-S47. |
Parsons, X. H. (2013). Reviving cell-based regenerative medicine for heart reconstitution with efficiency in deriving cardiac elements from pluripotent human embryonic stem cells (Editorial). Cardiol Pharmacol, 2(3), e112. |
Parsons, X. H. (2013). Cellular medicine for the heart-the pharmacologic utility and capacity of human cardiac stem cells. J Clin Exp Cardiolog S, 11, 2. |
Parsons, X. H. (2013). Exploring future cardiovascular medicine: heart precursors directed from human embryonic stem cells for myocardium regeneration. Cardiovascular Pharmacology: Open Access. |
Parsons, X. H. (2014). The openness of pluripotent epigenome-Defining the genomic integrity of stemness for regenerative medicine. International Journal of Cancer Therapy and Oncology, 2(1). |
Parsons, X. H. (2014). The designation of human cardiac stem cell therapy products for human trials. J Clin Trial Cardiol, 1(1), 2. |
Parsons, X. H. (2014). U.S. Patent No. 8,716,017. Washington, DC: U.S. Patent and Trademark Office. |
Parsons, X. H. (2013). Current state of regenerative medicine: moving stem cell research from animals into humans for clinical trials. Regen Med, 1(1), 1005. |
Yeh, D. C., & Chan, T. M. (2018). Therapeutics of Stem Cell Treatment in Anti-Aging and Rejuvenation. Stem Cell Discovery, 8(02), 13. |
Parsons, X. H. (2016). Human pluripotent stem cell-based PluriXcel technology platforms provide stem cell treatment development and manufacturing innovations for progressing to the clinic. J Regen Ther, 1(1), 1-8. |
Parsons, X. H. (2016). Journal of Regenerative Therapeutics Volume 1, Issue 1, October 2016, Pages 1â8. Journal of Regenerative Therapeutics, 1(1), 1-8. |
Parsons, X. H. (2016). U.S. Patent No. 9,428,731. Washington, DC: U.S. Patent and Trademark Office. |
Parsons, X. H. (2016). U.S. Patent Application No. 14/520,990. |
Navarro, X., Torres-EspÃn, A., Allodi, I., Santos, D., González-Pérez, F., Udina, E., & del Valle, J. (2016). Analysis of axonal growth in organotypic neural cultures. |
Farfán, L. F. T. (2013). La reprogramación en la obtención de células madre pluripotentes inducidas. ECIPeru: Revista del Encuentro CientÃfico Internacional, 10(1), 9-13. |
Farfán, L. F. T. (2013). La reprogramación en la obtención de células madre pluripotentes inducidas Reprogramming in obtaining induced pluripotent stem cells. Revista ECIPerú Volumen, 10(1). |
Srivastava, A., Dadheech, N., Vakani, M., & Gupta, S. (2018). Swertisin ameliorates diabetes by triggering pancreatic progenitors for islet neogenesis in Streptozotocin treated BALB/c mice. Biomedicine & Pharmacotherapy, 100, 221-225. |
Thakur, G., Lee, H. J., Jeon, R. H., Lee, S. L., & Rho, G. J. (2020). Small Molecule-Induced Pancreatic ?-Like Cell Development: Mechanistic Approaches and Available Strategies. International journal of molecular sciences, 21(7), 2388. |
Srivastava, A., Dadheech, N., Vakani, M., & Gupta, S. (2019). Pancreatic resident endocrine progenitors demonstrate high islet neogenic fidelity and committed homing towards diabetic mice pancreas. Journal of cellular physiology, 234(6), 8975-8987. |
Sassoli, C., Nosi, D., Tani, A., Chellini, F., Mazzanti, B., Quercioli, F., ... & Formigli, L. (2014). Defining the role of mesenchymal stromal cells on the regulation of matrix metalloproteinases in skeletal muscle cells. Experimental Cell Research, 323(2), 297-313. |
Sassoli, C., Frati, A., Tani, A., Anderloni, G., Pierucci, F., Matteini, F., ... & Meacci, E. (2014). Mesenchymal stromal cell secreted sphingosine 1-phosphate (S1P) exerts a stimulatory effect on skeletal myoblast proliferation. PLoS One, 9(9), e108662. |
Sassoli, C., Zecchi-Orlandini, S., Bani, D., & Formigli, L. (2013). Cardiac progenitor cells as target of cell and growth factor-based therapies for myocardial regeneration. J. Stem Cell Res. Ther, 9(004). |
Scott, C. M., Forster, C. L., & Kokkoli, E. (2015). Three-dimensional cell entrapment as a function of the weight percent of peptide-amphiphile hydrogels. Langmuir, 31(22), 6122-6129. |
Scott, C. M. (2016). Peptide-functionalized hydrogels for three-dimensional cell culture (Doctoral dissertation, University of Minnesota). |
POOK, T. MARTIN POOK. |
Menaa, F., Abdelghani, A., & Menaa, B. (2015). Graphene nanomaterials as biocompatible and conductive scaffolds for stem cells: impact for tissue engineering and regenerative medicine. Journal of tissue engineering and regenerative medicine, 9(12), 1321-1338. |
Kim, T. H., Lee, T., El-Said, W. A., & Choi, J. W. (2015). Graphene-based materials for stem cell applications. Materials, 8(12), 8674-8690. |
Shahrokhi, S., Daneshmandi, S., & Menaa, F. (2014). Tumor necrosis factor-?/CD40 ligand-engineered mesenchymal stem cells greatly enhanced the antitumor immune response and lifespan in mice. Human gene therapy, 25(3), 240-253. |
Martins, O., Matos, S., Figueiredo, M. H., Viegas, C., & Dias, I. (2014). Novel Nanocrystalline Hydroxyapatite for Bone Regeneration. J Regen Med 3: 1. of, 7, 14-17. |
Liu, M., Huang, F., Zhang, D., Ju, J., Wu, X. B., Wang, Y., ... & Zhao, Q. (2015). Heterochromatin protein HP1? promotes colorectal cancer progression and is regulated by miR-30a. Cancer research, 75(21), 4593-4604. |
Zhang, Y., Mignone, J., & MacLellan, W. R. (2015). Cardiac regeneration and stem cells. Physiological reviews, 95(4), 1189-1204. |
Gabisonia, K., & Recchia, F. A. (2018). Gene therapy for heart failure: new perspectives. Current heart failure reports, 15(6), 340-349. |
Piccoli, M. T., Gupta, S. K., & Thum, T. (2015). Noncoding RNAs as regulators of cardiomyocyte proliferation and death. Journal of molecular and cellular cardiology, 89, 59-67. |
Ponnusamy, M., Li, P. F., & Wang, K. (2017). Understanding cardiomyocyte proliferation: an insight into cell cycle activity. Cellular and Molecular Life Sciences, 74(6), 1019-1034. |
Liu, H., Zhang, Z., Wu, N., Guo, H., Zhang, H., Fan, D., ... & Liu, Y. (2018). Integrative analysis of dysregulated lncRNA-associated ceRNA network reveals functional lncRNAs in gastric cancer. Genes, 9(6), 303. |
Yuan, X., & Braun, T. (2017). Multimodal regulation of cardiac myocyte proliferation. Circulation research, 121(3), 293-309. |
Hu, E., Ding, L., Miao, H., Liu, F., Liu, D., Dou, H., & Hou, Y. (2015). MiR-30a attenuates immunosuppressive functions of IL-1?-elicited mesenchymal stem cells via targeting TAB3. FEBS letters, 589(24), 3899-3907. |
Sommese, L., Zullo, A., Schiano, C., Mancini, F. P., & Napoli, C. (2017). Possible muscle repair in the human cardiovascular system. Stem cell reviews and reports, 13(2), 170-191. |
Braga, L., Ali, H., Secco, I., & Giacca, M. (2021). Non-coding RNA therapeutics for cardiac regeneration. Cardiovascular research, 117(3), 674-693. |
Xiao, J., Liu, H., Cretoiu, D., Toader, D. O., Suciu, N., Shi, J., ... & Li, X. (2017). miR-31a-5p promotes postnatal cardiomyocyte proliferation by targeting RhoBTB1. Experimental & molecular medicine, 49(10), e386-e386. |
Raso, A., & Dirkx, E. (2017). Cardiac regenerative medicine: at the crossroad of microRNA function and biotechnology. Non-coding RNA research, 2(1), 27-37. |
Sadiq, S., Crowley, T. M., Charchar, F. J., Sanigorski, A., & Lewandowski, P. A. (2017). Micro RNA s in a hypertrophic heart: from foetal life to adulthood. Biological Reviews, 92(3), 1314-1331. |
Soler-Botija, C., Forcales, S. V., & GenÃs, A. B. (2020, January). Spotlight on epigenetic reprogramming in cardiac regeneration. In Seminars in cell & developmental biology (Vol. 97, pp. 26-37). Academic Press. |
Wu, L., Yang, K., Gui, Y., & Wang, X. (2020). Nicotine-upregulated miR-30a arrests cell cycle in G1 phase by directly targeting CCNE2 in human periodontal ligament cells. Biochemistry and Cell Biology, 98(3), 354-361. |
Dong, X., Dong, X., Gao, F., Liu, N., Liang, T., Zhang, F., ... & Chen, J. (2021). Non?coding RNAs in cardiomyocyte proliferation and cardiac regeneration: Dissecting their therapeutic values. Journal of Cellular and Molecular Medicine, 25(5), 2315-2332. |
Yeh, D. C., & Chan, T. M. (2018). Therapeutics of Stem Cell Treatment in Anti-Aging and Rejuvenation. Stem Cell Discovery, 8(02), 13. |
MACLELLAN, W. R., & Danny, E. N. (2016). |
Raso, A., & Dirkx, E. (2017). Non-coding RNA Research. |
Bongiovanni, C., Sacchi, F., Da Pra, S., Pantano, E., Miano, C., Morelli, M. B., & D'Uva, G. (2021). Reawakening the Intrinsic Cardiac Regenerative Potential: Molecular Strategies to Boost Dedifferentiation and Proliferation of Endogenous Cardiomyocytes. Frontiers in Cardiovascular Medicine, 1283. |
Yang Qian, Gui Yonghao, & Li Qiang. (2016). MicroRNA regulation of DNA methylation in congenital heart disease and its application prospects. Chinese Journal of Pathophysiology , 32 (11), 2101-2105. |
Akane Sakaguchi, & Ko Kimura. (2018). Elucidation of cardiomyocyte proliferation control mechanism. Heart , 50 (12), 1276-1282. |
Sych, N. S., Ivankova, O. V., Klunnyk, M. O., Matiyashchuk, I. G., Sinelnyk, A. A., Demchyk, M. P., ... & Siniscalco, D. (2015). Fetal stem cells are effective in the treatment of Grade I and II respiratory failure in amyotrophic lateral sclerosis and muscular dystrophy. Translational Neuroscience and Clinics, 1(1), 10-16. |
Dutta, R. C., & Dutta, A. K. (2018). 3D Cell Culture: Fundamentals and Applications in Tissue Engineering and Regenerative Medicine. CRC Press. |
Pantelic, M. N., & Larkin, L. M. (2018). Stem cells for skeletal muscle tissue engineering. Tissue Engineering Part B: Reviews, 24(5), 373-391. |
Sarrafian, T. L., Bodine, S. C., Murphy, B., Grayson, J. K., & Stover, S. M. (2018). Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Veterinary Surgery, 47(4), 524-535. |
Iannotti, F. A., Pagano, E., Guardiola, O., Adinolfi, S., Saccone, V., Consalvi, S., ... & Di Marzo, V. (2018). Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nature communications, 9(1), 1-13. |
Sohn, J., Lin, H., Fritch, M. R., & Tuan, R. S. (2018). Influence of cholesterol/caveolin-1/caveolae homeostasis on membrane properties and substrate adhesion characteristics of adult human mesenchymal stem cells. Stem cell research & therapy, 9(1), 1-15. |
Oh, S., Jung, S. H., Seo, H., Min, M. K., Kim, B., Hahn, Y. K., ... & Choi, S. (2018). Magnetic activated cell sorting (MACS) pipette tip for immunomagnetic bacteria separation. Sensors and Actuators B: Chemical, 272, 324-330. |
Syverud, B. C., Lin, E., Nagrath, S., & Larkin, L. M. (2018). Label-free, high-throughput purification of satellite cells using microfluidic inertial separation. Tissue Engineering Part C: Methods, 24(1), 32-41. |
Fish, K. D., Rubio, N. R., Stout, A. J., Yuen, J. S., & Kaplan, D. L. (2020). Prospects and challenges for cell-cultured fat as a novel food ingredient. Trends in food science & technology, 98, 53-67. |
Rodriguez, B. L., Nguyen, M. H., Armstrong, R. E., Vega-Soto, E. E., Polkowski, P. M., & Larkin, L. M. (2020). A comparison of ovine facial and limb muscle as a primary cell source for engineered skeletal muscle. Tissue Engineering Part A, 26(3-4), 167-177. |
Li, L., Liu, G., Timashev, P., Sun, X. S., Criswell, T., Atala, A., & Zhang, Y. (2019). Biofabrication of tissue-specific extracellular matrix proteins to enhance the expansion and differentiation of skeletal muscle progenitor cells. Applied Physics Reviews, 6(2), 021309. |
Careccia, G., Colombo, F., Tirone, M., Agresti, A., Bianchi, M. E., Zambrano, S., & Vénéreau, E. (2019). Exploiting live imaging to track nuclei during myoblast differentiation and fusion. JoVE (Journal of Visualized Experiments), (146), e58888. |
Lin, E. (2017). High throughput microfluidic labyrinth for the label free isolation of CTCs for single cell gene expression profiling (Doctoral dissertation). |
Yeh, D. C., & Chan, T. M. (2018). Therapeutics of Stem Cell Treatment in Anti-Aging and Rejuvenation. Stem Cell Discovery, 8(02), 13. |
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi