Research Article, Androl Gynecol Curr Res Vol: 4 Issue: 1
IVF-ICSI Split Insemination Reveals those Cases of Unexplained Infertility Benefitting from ICSI Even when the DNA Fragmentation Index is Reduced to 15% or Even 5%
| Kamarul B Mustafa1,2, John L Yovich1*, Nicole Marjanovich1, Steven J Yovich1 and Kevin N Keane1,3 | |
| 1PIVET Medical Centre, Perth, Western Australia, Australia | |
| 2Fertility Centre, Obstetrics & Gynaecology Department, Kulliyyah of Medicine, International Islamic University Malaysia (IIUM), Pahang, Malaysia | |
| 3School of Biomedical Science, Curtin Health Innovation Research Institute, Bioscience, Curtin University, Australia | |
| Corresponding author : John L Yovich PIVET Medical Centre, Perth, Western Australia, Australia, Tel: 9422 5400 E-mail: jlyovich@pivet.com.au |
|
| Received: September 09, 2015 Accepted: March 31, 2016 Published: March 31, 2016 | |
| Citation: Mustafa KB, Yovich JL, Marjanovich N, Yovich SJ, Keane KN (2016) IVF-ICSI Split Insemination Reveals those Cases of Unexplained Infertility Benefitting from ICSI Even when the DNA Fragmentation Index is Reduced to 15% or Even 5%. Androl Gynecol: Curr Res 4:1. doi:10.4172/2327-4360.1000145 |
Abstract
IVF-ICSI Split Insemination Reveals those Cases of Unexplained Infertility Benefitting from ICSI Even when the DNA Fragmentation Index is Reduced to 15% or Even 5%
Purpose The aim of the study was to assess the fertilization rate (FR) of randomized sibling oocytes inseminated by conventional IVF or ICSI in couples with unexplained infertility. Methods The 16-month study was conducted at an established private IVF facility. Oocytes recovered from couples with normal semen parameters and normal DNA fragmentation index (DFI; <30%), were randomly allocated to IVF or ICSI and the FR (2PN/MII) was assessed. Pregnancy outcome following embryo transfers were analyzed with regards to either IVF-embryo vs. ICSI-embryo, and in relation to DFI levels. Results Of 585 oocytes retrieved from 38 patients, 463 were mature (MII). The ICSI group generated a significantly higher number of 2PN embryos with a mean FR of 83.4% vs. 67.6% (p<0.05). There were no cases of complete fertilization failure (CFF) in the ICSI group, but there were 7.9% in the IVF group. The significant difference of FR was observed only when the DFI level was ≥ 15% and if such cutoff was applied, the CFF cases would be reduced to 2.6%. Of the 30 patients who had fresh embryo transfers performed, the ICSI group showed a higher pregnancy rate (69.2% vs. 58.8%; N.S.) with a significantly higher mean DFI value in the non-pregnant group (p<0.05). Conclusions IVF-ICSI split insemination can reveal those cases which will benefit from ICSI even where semen parameters and DFI are normal; however if the DFI is reduced to a 15% cut-off level, the rate of CFF will be minimized, but not completely excluded, even at 5%..
Keywords: IVF-ICSI split; Fertilization rate; Fertilization failure; DNA fragmentation; DFI; Embryo utilization
Keywords |
|
| IVF-ICSI split; Fertilization rate; Fertilization failure; DNA fragmentation; DFI; Embryo utilization | |
Introduction |
|
| Complete fertilization failure (CFF) or low fertilization rates despite a good yield of oocytes in conventional in vitro fertilization (IVF) cycles could have devastating psychological and financial effects on the patients undergoing treatment as well as affecting the reputation of both the embryologist and the treating clinician. Intracytoplasmic sperm injection (ICSI) is believed to produce a better chance at fertilization because of the direct manual sperm injection into the oocyte. However, it was not favoured initially because the procedure overrides biological safeguards that typically prevent sperm with damaged deoxyribonucleic acid (DNA) fertilizing via spontaneous pregnancy or conception after conventional IVF. This worry has been largely proved unfounded because it has been noted that there was a significant decrease in implantation and pregnancy rates following the use of sperm with high DNA fragmentation (DFI), which indicated that the damaged paternal genome is selected against embryonic development [1]. There is also a lack of evidence for an increased incidence of genetic damage or major congenital malformations among children born after ICSI [2-4]. Those found to have increased risk were believed to be related to parental background factors that required the use of ICSI in the first place, not due to the actual technique [5,6]. | |
| ICSI is usually reserved for patients with abnormal sperm parameters. However, split insemination of oocytes by both IVF and ICSI is strongly encouraged in our centre even for couples with normozoospermic sperm analysis and DFI. This followed an historic case at our clinic, where CFF occurred after conventional IVF of 18 oocytes derived from one of our patients (who had poorly explained infertility; regular menstrual cycles with polycystic ovaries), despite normal sperm parameters based on WHO 2010 classification and normal DFI of 16%. She fortunately conceived successfully two months later (and had a singleton live birth of a baby boy) following all-ICSI fertilization of oocytes. Therefore, the current study was aimed at auditing the effect of the change in this protocol, and to analyze the outcome of IVF-ICSI split in normozoospermic patients with normal DFI. The primary outcome measure was fertilization rate. The secondary outcome measures were embryo utilization rate and pregnancy rate, in relation to DFI. | |
Materials and Methods |
|
| Patient selection | |
| The study period was from 16th of June 2014 until 16th of October 2015 (16 months) inclusive. Couples were included into the study if they were undertaking IVF for the first time, the male partners had normal semen parameters as well as DFI of <30%, and there were at least four mature oocytes following retrieval. (All cases with <4 mature oocytes had routine all-ICSI according to existing clinic protocols). The study period followed soon after the case mentioned at introduction; and was designed to enable the development of an improved protocol to avoid cases of unexpected CFF. The cut-off date was chosen to capture all pregnancy outcomes at least up to seven weeks gestation at the time of data analysis. The complete dataset was extracted from our extensive database using Filemaker Pro 12 (Apple, USA) and which undergoes regular validation checks. | |
| All of the male partners had normal semen quality based on the WHO 2010 criteria [7] with sperm count of ≥ 15×106/ml, ≥ 32% progressive motile sperm and ≥ 4% morphologically normal sperm cells. Patients with male partners with abnormal semen quality were excluded. The male partners had their semen analyzed for DFI using the Halo test (Halosperm Halotech® DNA) [8]. The cut off points for the DFI is taken as 30% [9] as DFI levels beyond this value are not compatible with the initiation and maintenance of a term pregnancy [10,11]. | |
| Ovarian stimulation | |
| All patients had ovarian stimulation with either antagonist or flare protocol that have been clearly described elsewhere [12,13]. The selection of the stimulation protocol was at the discretion of the clinician, but the preference was usually the antagonist regimen for younger women with higher antral follicular count (AFC) ratings and the flare regimen was more commonly used for older women with low AFC ratings. | |
| Sperm preparation, oocyte retrieval, insemination and embryo grading | |
| The IVF-ICSI split was conducted by inseminating/injecting sibling oocytes using conventional IVF or ICSI from the same semen sample in all patients. The semen sample on the day of oocytes retrieval was prepared using Pure-sperm (Density gradient) or Swim- Up method. The culture medium used was either Vitrolife (G-seriesTM Culture Media, GÖtenberg, Sweden), Cook (Cook Medical Inc. Sydney IVF Embryo Culture Media, Bloomington, USA) or Quinns (Origio, a CooperSurgical Company, Quinns AdvantageTM Culture Media, Denmark). Sibling oocytes will be cultured in the same culture medium. After completion of oocyte retrieval, the oocytes were then graded [14] and randomly allocated by one of the study-group embryologists based on the grades (which included follicle size and cumulus-oocyte features) into both the IVF and ICSI groups. Oocytes were pre-incubated at 37°C for 3-6 hours before insemination. | |
| The cumulus cells were then removed in the ICSI group, and only the metaphase II (MII) oocytes were GÖtenberg injected with sperm. Embryos were checked for pronuclei (PN) 16-18 hours later. The number of MII oocytes in the IVF group was calculated retrospectively based on this PN check. All germinal vesicle (GV) and metaphase I (MI) oocytes noted at this stage were considered immature, all embryos with PN were considered as MII at the time of insemination. | |
| The fertilization rate was expressed as the percentage of twopronuclear (2PN) oocytes generated from the number of MII oocytes inseminated (2PN/MII×100). The utilization rate was defined by the percentage of embryos deemed suitable for transfer or cryopreservation arising from the total number of 2PN oocytes generated (Tables 1 and 2). | |
| Table 1: Demographics for women undergoing IVF-ICSI split insemination. | |
| Table 2: Demographics and sperm DNA fragmentation (DFI) for male partners undergoing IVF-ICSI split insemination. IVF-ICSI: in vitro fertilization–intracytoplasmic sperm injection. SD: Standard deviation. | |
| Embryo transfer and pregnancy | |
| All embryo transfers were undertaken either as Day 3 cleavage embryo or Day 5 blastocyst embryo as single embryo transfers (SET). Minimum criteria for a Day-3 transfer included the presence of ≥ 6 blastomeres with grading criteria ≥ 2.0 (14). The embryos were cultured further to blastocysts (Day 5 or 6) if there were ≥ 4 suitable embryos at Day 3. Otherwise embryo transfer was performed at Day 3. The clinic protocol includes SET for all cases undergoing their first IVF-ICSI split procedure and the best embryo was always selected for transfer in this study period. | |
| Luteal support was as per our long-standing protocol based upon the number of oocyte retrieved. HCG 500-1000 units daily (when oocyte numbers were between 5 and 15) on days 4, 7, 10 and 13 where day 0 is the day of oocyte retrieval. Pessaries (Progesterone or combined estradiol/progesterone pessary; compounded products) may also be added on the day of retrieval [15]. When there was ≥ 15 oocytes retrieved, cabergoline 1 mg daily for 10 days would be added. In the case of ≥ 20 oocytes retrieved, no HCG would be given, and support was achieved using the pessaries. In the case of freeze all embryo cycles, Provera 10 mg daily for 12 days would be given from the day of retrieval [13]. Midluteal serum hormonal check (estradiol and progesterone) signified whether additional support hormones were required. | |
| Clinical pregnancy was defined as a visible gestational sac at 7 weeks gestation or if miscarriage occurred, pregnancies were confirmed by the histopathological report of presence of chorionic villi. Otherwise pregnancies were classified as biochemical only and excluded from the data analysis. | |
| Statistical analysis | |
| SPSS-22 Software was used for the statistical analysis. The Paired-samples T test was used to compare means after normality check with Q-Q plot. Independent sample T test and Fisher’s Exact X2 test were used when data were analyzed in the treatment outcome following embryo transfer of either following IVF or ICSI. Statistical significance was considered when the p values were <0.05. | |
Results |
|
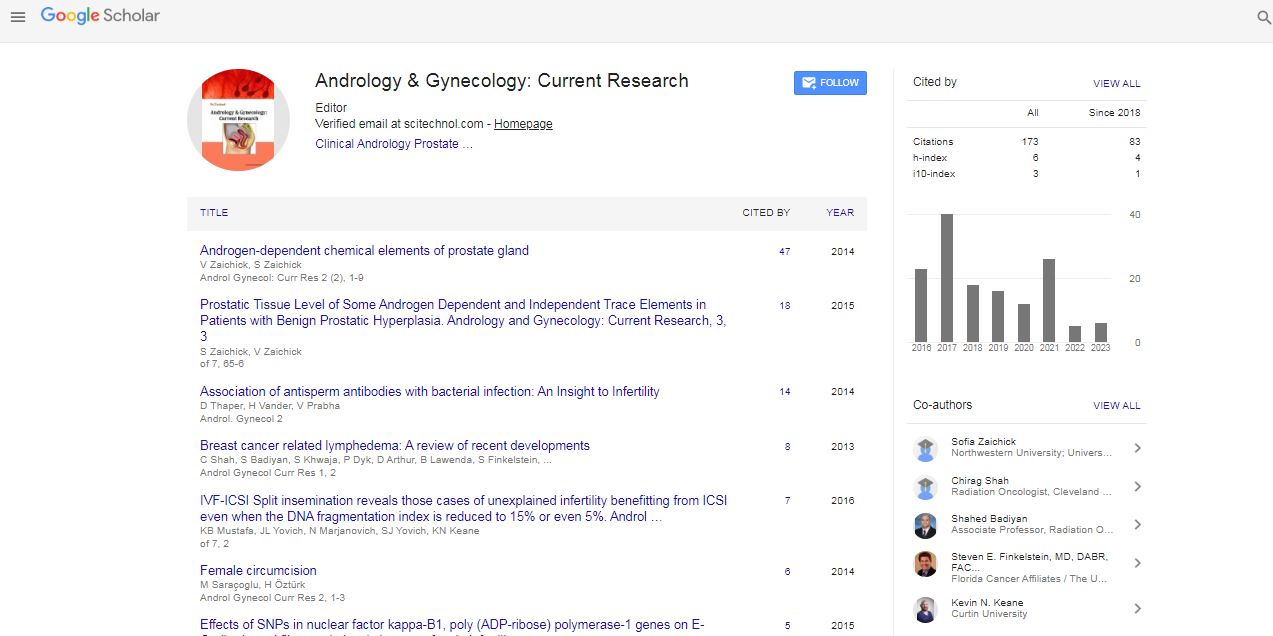
| There was a total of 585 oocytes collected, 463 of which were mature (MII) oocytes (Figure 1 and Table 3). There was no significant difference in the number of MII oocytes allocated between the IVF and ICSI group. However, the ICSI group generated a significantly higher number of 2PN embryos with mean fertilization rate of 83.4%, as opposed to the IVF group with only 67.6% (p<0.05). None of the ICSI group had CFF, but there were three instances in the IVF group. In these three cases, the DFI levels were 18%, 16%, and 4.5%. Furthermore, the embryo utilization rate in the ICSI group was also higher (65.4% vs. 55.8%) though not significant statistically. When sub-analysis of fertilization and embryo utilization rates were performed in the IVF group with fertilization rates ≤50%, the fertilization rate differences became more significant when compared with the corresponding rate in the ICSI group (80.7% vs. 30.0%, p<0.001). However, there was no significant difference in the embryo utilization rate. | |
| Figure 1: Flow Chart of IVF-ICSI split cycles: recruitment and outcome. | |
| Table 3: Fertilization and embryo utilization rate in sibling oocytes following IVF-ICSI Split. | |
| All but one patient (97.4%) had their embryos successfully cultured to the blastocyst stage and had a Day 5 embryo transfer or had their embryos cryopreserved as blastocysts. The only patient transferred on Day 3 had four 2PN embryos but only three developed to our minimum Day 3 criteria; one was transferred and the remaining 2 were submitted to further culture but neither developed to blastocysts of suitable quality for cryopreservation. There was also a significant difference in the FR when the DFI grouping was ≥ 15%; p<0.05 and if such cutoff was applied the cases of CFF would have reduced to 2.6%. | |
| Of the 38 patients, 8 had all their embryos cryopreserved (i.e. no transfer procedure undertaken) due to the risk of ovarian hyperstimulation syndrome (OHSS) (Figure 1). Of the 30 who had embryo transfers performed, there were no significant differences in the mean age or mean BMI of the women, or the mean age of the male partners between the IVF and ICSI groups (Table 4). In addition, there was also no significant difference in the mean sperm DFI between the two groups. Conversely, the ICSI group had a higher pregnancy rate of 69.2% (9/13) versus 58.8% (10/17) for the IVF group, but this did not reach statistical significance. Further evaluation comparing clinical and biochemical pregnancies were hindered by the small number of patients, as there were only two biochemical pregnancies. There were also only two miscarriages associated with the clinical pregnancies – again the number was too small for comparative study. However, one of the miscarriages had the highest DFI level of 22.5%. | |
| Table 4: Treatment outcomes of embryo transfers following IVF-ICSI Split. | |
| When the DFI was divided further into several categories (Table 5) a trend was noted of increasing difference in the FR between the IVF and ICSI groups reaching statistical significance with DFI ≥15% (48.6% vs. 81.5%; p<0.05). However, once embryos were generated their utilization rates were not significantly different. Analyzing the DFI levels with respect to those cases achieving pregnancies (Table 6), there was a significantly lower mean DFI level (8.6 vs. 13.6; p<0.05). Those pregnancies arising in the DFI ≥ 15 group (n=2) resulted in either biochemical pregnancy or miscarriage.However, 88.2% (15/17) pregnancies in DFI <15 ended successfully with singleton live birth or were still ongoing at the time of data collection. | |
| Table 5: IVF-ICSI split outcomes in relation to sperm DFI range. | |
| Table 6: Pregnancy outcomes in relation to sperm DFI range. | |
| The chances of avoiding CFF or having a poor fertilization rate (FR ≤ 50%) are shown in Table 7 depicting the respective positive predictive value (PPV) according to DFI levels. At DFI ≤ 30, the PPV for avoiding CFF from IVF was 92.1% but reduced to 71.1% when inclusive of poor fertilization (FR ≤ 50%). For the ICSI group, the PPV values were excellent at 100% and 97.4% respectively. At DFI ≤ 15, the PPV for avoiding CFF in the IVF group is 97.4% reducing to 81.6% when inclusive of poor fertilization. For the ICSI group, the PPV remains at the same high values, being 100% for avoidance of CFF and 97.4% for avoidance of poor fertilization. At DFI limit of ≤ 5, the PPV value for avoiding CFF in the IVF group was 97.4% reducing to 92.1% when inclusive of poor fertilization. For the ICSI group, the PPV values for avoiding both CFF and poor fertilization were 100% for both. | |
| Table 7: Positive predictive value to avoid CFF or poor fertilization related to DFI. | |
Discussion |
|
| Our study of IVF-ICSI split insemination of sibling oocytes randomized from patients with mainly unexplained fertility and whose male partner had normal semen quality as well as DFI, found that ICSI oocytes had a significantly higher fertilization rate than those inseminated by conventional IVF. The randomized allocation and distribution ensured there was no difference in the mean number of mature oocytes allocated to each group. This outcome was in agreement with earlier findings [16,17]. We also found that those in the conventional IVF group with poor fertilization had vastly lower fertilization rates than the ICSI group (30.0% vs. 80.7%, p<0.001). This further emphasized the importance of IVF-ICSI split in couples with normal semen parameters. | |
| For most IVF programmes, the incidence of CFF was reported to occur in 5-10% of IVF cycles and 2-3% of ICSI cycles [18]. In our study, the CFF rate for IVF was 7.9%, being in agreement with the previous study, but we had no cases of CFF in the ICSI group. Even though the CFF occurrences in the IVF group can be considered as small, to the patients who had to bear the consequences, there is inevitably a huge emotional and financial impact. It was also noted that two of the CFF cases had DFI of ≥ 15, and the other other one had DFI <15. Adoption of the IVF-ICSI split insemination model may therefore help eliminate fertilization failures and avoid the loss of invaluable biological time, along with the cost of failed cycles, and the psychological pain of repeated conventional IVF failure. It also identifies those cases requiring all-ICSI in future as the technique for assured fertilization, above other available diagnostic processes (semen parameters and DFI test). | |
| However regardless of the technique by which embryos were generated (IVF or ICSI), their utility was similar. An apparently higher embryo utilization rate for ICSI did not reach statistical significance (65.4% vs. 55.8%; NS). Similarly, despite an apparently higher pregnancy rate with ICSI embryos, the chance of pregnancy following SET of the best quality embryo showed no significant difference (69.2% vs. 58.8%; NS). However other studies have linked DNA damage with a decreased chance of clinical pregnancies from IVF [19,20] as well as significantly increasing the risk of pregnancy loss after both IVF and ICSI [21]. Recruitment of a larger cohort of patients would provide more power to properly address this issue as there may be relevance for miscarriages with the implication that IVF-ICSI split is more sensitively identifying DNA damage. | |
| Our study found that when the DFI was ≥ 15 there was a significant reduction in the fertilization rate in the IVF vs. ICSI group of the sibling oocytes (48.6% vs. 81.5%, p<0.05). There was also a significantly higher mean DFI level in the non-pregnant group (13.6 vs. 8.6, p<0.05). Even though these levels were still within the previously designated ‘normal’ limits of <30%, it indicated that the higher the DFI, the higher the chance of pregnancy failure and the lower the chance of livebirth. This is of potential relevance when considering that one of the miscarriages observed in our study had the highest DFI value of 22.5%. It is also important to note that all good outcome pregnant cases had DFI of <15. None of the pregnancies with DFI ≥ 15 ended favourably. In contrast, 88.2% pregnancies with DFI <15 resulted in singleton live birth or were still ongoing at the time of data collection. These results indicated a lower cut–off DFI level would be more relevant for the indication of ICSI – using a value of 15% instead of the usual 30%. If such cut-off was applied the PPV for avoiding cases of CFF and poor fertilization would have improved from 71.1% to 81.6% rising to 97.4% for avoidance of CFF alone. However, our study showed that no DFI level could provide a 100% PPV, even with DFI<5. Patients should therefore be informed that ICSI is required at all DFI levels to avoid CFF completely. Our study showed a PPV of 100% for fertilization with ICSI, but other larger studies imply a PPV of 98% may be more realistic [18]. | |
| There is therefore an obvious clinical indication for the evaluation of sperm DNA damage prior to IVF or ICSI – which may help in the counseling of patients with regards to the success rate and practicing a healthy life style, especially in avoiding smoking [22-24] which has a known association with elevated DFI levels. Our current recommendation is to classify the DFI into different sub-groups with significance explained as denoted in Table 8. This would use DFI ≥ 15 as the recommended cut-off point for ICSI. DFI level of <15 may be suitable for IVF only – but bearing in mind that IVF-ICSI split should still be considered for all cases in the first fresh IVF cycle because the chance of CFF was not excluded even at a very low level of DFI <5. | |
| Table 8: Sperm DNA fragmentation index (DFI): revised classification. | |
Conclusion |
|
| We conclude that IVF-ICSI split can reveal those cases which will benefit from ICSI even where semen parameters and DFI are normal. However if the DFI level is reduced to a cut-off level of <15%, the rate of CFF will be minimized, but not completely excluded, even at <5%. IVF units wishing to minimize costs should consider that the IVFICSI model described has benefits over DFI testing in identifying all cases which would benefit from ICSI. | |
Acknowledgments |
|
| This study was not supported by any research grant. The authors would like to express gratitude to all the staff of the IVF facility involved directly or indirectly in generating the data and ensuring the success of the research. | |
Ethical Approval |
|
| All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard. The facility is accredited with both the self-regulatory National Australian Reproductive Technology Committee (RTAC) as well as the Reproductive Technology Council (RTC) of Western Australia (established under the Western Australian Human Reproductive Technology Act, 1991). These agencies monitor all activities conducted at the IVF centre and demand oversight by an NHMRC-constituted Institutional Ethics Committee who endorse all clinical and laboratory protocols. Reporting of the data was approved under Curtin University Ethics Committee approval no. RD-25-10 general approval for retrospective data analysis 2011, updated 2015. | |
References |
|
|
|
 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi