Research Article, J Pharm Drug Deliv Res Vol: 4 Issue: 4
Deformable Liposomes for the Transdermal Delivery of Piroxicam
| Helena Ferreira1,2*, Artur Ribeiro2, Raquel Silva2 and Artur Cavaco-Paulo2* | |
| 1CESPU, Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde, Rua Central de Gandra, 1317, 4585-116 Gandra PRD, Portugal. | |
| 2CEB -Centre of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057, Braga, Portugal | |
| Corresponding author's : Artur Cavaco-Paulo CEB - Centre of Biological Engineering, Universidade do Minho, Campus de Gualtar, 4710-057, Braga, Portugal Tel:+351253510271; Fax: +351253510293; E-mail: artur@det.uminho.pt |
|
| Helena Ferreira CESPU, Instituto de Investigação e Formação Avançada em Ciências e Tecnologias da Saúde, Rua Central de Gandra, 1317, 4585-116 Gandra PRD, Portugal Tel:+351 224 157 136; Fax: +351 224 157 102; E-mail: helenaferreira@dep.uminho.pt |
|
| Received: September 09, 2015 Accepted: November 18, 2015 Published: November 23, 2015 | |
| Citation: Ferreira H, Ribeiro A, Silva R, Cavaco-Paulo A (2015) Deformable Liposomes for the Transdermal Delivery of Piroxicam. J Pharm Drug Deliv Res 4:4. doi:10.4172/2325-9604.1000139 |
Abstract
Deformable Liposomes for the Transdermal Delivery of Piroxicam
Abstract
Objective: Deformable liposomes have been used to improve drugs transdermal delivery. These vesicular systems were employed to deliver piroxicam through the skin as a mean to treat inflammatory diseases and avoid undesired side-effects.
Methods: Deformable liposomes, composed by egg-yolk phosphatidylcholine, sodium cholate and α-tocopherol, were prepared by the thin film hydration method followed by extrusion. Piroxicam was included into the lipid bilayer or in the aqueous phase using inclusion complexes of piroxicam with β-cyclodextrin. After characterization, it was evaluated their in vitro permeation using Franz diffusion cells with polysulfone membranes or pig skin.
Results: The entrapment of piroxicam in the aqueous compartment, through the use of β-cyclodextrin inclusion complexes, enabled higher entrapment efficiency (63.27% more than when entrapped in the lipid bilayer). The optimized deformable liposomes population were homogeneous (PDI < 0.1) in terms of size (108.93 ± 3.74 nm) and presented a spherical shape. Size stability studies demonstrated that the vesicles were stable along two months of storage. In vitro permeation studies using Franz diffusion cells and polysulfone membranes showed that the vesicles own enough deformability to pass through pores smaller than their own size in a percentage ≈ 45%. Furthermore, a constancy of their diameter and morphology was verified after pores passage. In the experiments performed with pig skin, the permeation of the deformable liposomes incorporating piroxicam β-cyclodextrin complexes decrease considerably. After 24 h of diffusion, only 1.1-3.2 % of the initial population reached the liquid receptor as result of the presence of the stratum corneum which is the main barrier of the skin. Nevertheless, the histological studies demonstrated that deformable liposomes were uniformly distributed on the skin structure and thus were able to achieve a percutaneous permeation of their content.
Conclusion: The results support the possibility to use this formulation on the topical treatment of inflammatory conditions.
Keywords: Deformable liposomes; Non-steroidal anti-inflammatory drug; β-cyclodextrin; Pig skin; Skin permeation; Franz diffusion cells; Inflammatory conditions
Keywords |
|
| Deformable liposomes; Non-steroidal anti-inflammatory drug; β-cyclodextrin; Pig skin; Skin permeation; Franz diffusion cells; Inflammatory conditions | |
Introduction |
|
| Piroxicam, a non-steroidal anti-inflammatory drug (NSAID) of the oxicams group, is effective in the treatment of rheumatoid arthritis, osteoarthritis and local inflammations [1,2]. However, this drug, known as cyclooxygenase-1 inhibitor, presents gastrointestinal side effects limiting its oral therapy [3-5]. Therefore, topical therapy is an appealing alternative to circumvent these side effects highlighting at same time others advantages related with this route of administration, as first passage metabolism avoidance [6]. Nevertheless, it is necessary a formulation that ensures a suitable percutaneous penetration into viable skin, since the stratum corneum provides the major obstacle for the transdermal permeation of a drug [7,8]. In this work it is intended to develop a formulation based in deformable liposomes for skin delivery of piroxicam in order to increase its therapeutic index. Deformable liposomes will allow the percutaneous penetration of the anti-inflammatory drug, diminishing the amount of drug necessary to achieve a therapeutic effect while minimizing toxic effects when compared with oral administration. One of the most promising topical vesicular drug delivery systems are liposomes [7-9]. In fact, being biocompatible, biodegradable and non-irritant to the skin [10], make liposomes very attractive to be used as carriers for topical therapy. However, to penetrate deeper into the skin it is necessary to incorporate an edge activator into the phospholipid bilayer [7-9]. The vesicles with this composition, known as deformable liposomes (Transfersomes®), were developed by Cevc and Blume [11]. Deformable liposomes present higher elasticity than conventional liposomes, allowing their penetration into the pores with 1/5 of their size [10-12]. In fact, the extremely high and stress-dependent adaptability permits that deformable liposomes can squeeze between the cells in the stratum corneum, without irreversible disruption, despite their higher diameter than pores in the skin [12]. The improvement of skin delivery of various drugs by this type of liposomes is reported by several studies present in the literature. Indeed, deformable liposomes demonstrated to provide a higher skin permeation of the material entrapped into their structure, as shown for the natural compound asiaticoside. This compound when incorporated into the liposome formulation had an in vitro skin permeation 10-fold higher than its aqueous solution with a concomitant increase of its therapeutic effect both in vitro and in vivo. Similar results were obtained for oestradiol [13]. The deformable liposomes incorporating this hormone significantly improve its in vitro skin delivery comparatively to its saturated aqueous solution. In another study [14], the authors shown that deformable liposomes were more effective in the delivery of methotrexate through the pig skin than conventional liposomes and an aqueous solution of that drug. The same comparison was made for meloxicam and one more time the meloxicam-loaded deformable liposomes lead to a greater skin permeation of the NSAID [15]. Additionally, it was demonstrated that the improvement of skin delivery of drugs, as ketotifen, by deformable liposomes might be related with the penetration increasing effect and also with the undamaged vesicle permeation into the stratum corneum [16]. Moreover, the incorporation of drugs in deformable liposomes can lower the therapeutic dosage, increase biological potency and lower frequency of application, as obtained for hydrocortisone and dexamethasone [17]. In this work, as just referred, deformable liposomes were used to deliver piroxicam across skin. Sodium cholate was used as edge activator, since it is frequently used for this purpose [18]. In the composition of the deformable liposomes it was also included α-tocopherol to protect the delivery system formulated against oxidation. To produce deformable liposomes, first it was used the thin film hydration method [19], which is widely used to prepare multilamellar vesicles (MLVs). In this method, the organic solvents used to dissolve the lipids are evaporated in order to obtain a dry lipid film onto the wall of a round-bottomed flask. The lipid film formed is then hydrated with an appropriate aqueous solution and the vortex mixing of the obtained mixture allows the formation of MLVs. To produce deformable unilamellar liposomes (LUVs) it is possible to apply different methodologies, such as extrusion, high pressure homogenization and sonication. Extrusion through polycarbonate membranes with a defined pore diameter was the method used in this work to prepare LUVs. In fact, this method presents some advantages regarding the other cited methods, such as avoidance of oxidation as well as the production of a more homogeneous population in terms of size. The incorporation of piroxicam in these vesicles was first made in the lipid bilayer, due to its hydrophobicity, and then through the use of β-cyclodextrin inclusion complexes in the aqueous compartment of the liposomes. It is well documented that piroxicam form an inclusion complex with β-cyclodextrin [20-22], and that complexation can increase the aqueous solubility of the NSAID in the order of five times [23]. The two strategies used to entrap the NSAID in the deformable liposomes were performed to verify which approach had higher piroxicam entrapment efficiency. The incorporation of the lipophilic drugs in the aqueous compartment instead in the lipid bilayer of the liposomes has advantages, such as the delay of the drug release after administration and an increase of the amount of the entrapped drug since it is not limited in terms of drug to lipid ratio [24,25]. Cyclodextrins can form complexes with several drugs and consequently have been used to increase the solubility and stability of the hydrophobic drugs [25,26]. Additionally, as no covalent bounds are present in the inclusion complex, it can occur a rapid dissociation of the drug complexed with the cyclodextrin [25]. Therefore, in an unique system it is possible to combine the advantages of cyclodextrin inclusion complexes with those of the deformable liposomes [27]. For example, this system was used to incorporate betamethasone and significantly improve its entrapment efficiency. Moreover, the developed vesicles present a good stability and provide higher in vitro permeation of the entrapped glucocorticoid [27]. | |
| After optimization of liposome preparation, in vitro permeation experiments using Franz diffusion cells were performed. First the penetration ability of the deformable liposomes was evaluated using polysulfone membranes. To confirm if these carriers are promising to deliver the piroxicam into the deepest skin layers, pig skin was used as a model. The determination of size and morphology after polysulfone membrane passage was performed to monitor possible changes in the deformable liposomes structure. Histological studies were done as a mean to check the distribution of the deformable liposomes in the skin. Last, it was measured the amount of phospholipid and the fluorescent compound fluorescein 5(6)-isothiocyanate (FITC) deposited in the pig skin. | |
Materials and Methods |
|
| Materials | |
| The chemical reagents, unless otherwise noted, were purchased from Sigma-Aldrich (Spain) and all were used as supplied. The pig skin was generously supplied by Matadouro Central de Entre Douro e Minho (Portugal). For histology studies the Cryoprotectant Tissue- Tek Oct compound was purchased from Sakura (Netherland) and the Thermo Scientific Shandon Immu-Mount was purchased from Fisher Healthcare (UK). | |
| Preparation of deformable liposomes | |
| Deformable liposomes were prepared considering the thin film hydration method and according the previous optimized sodium cholate concentration [13,18]. Briefly, egg-yolk phosphatidylcholine (EPC) and sodium cholate mixed in a proportion of 86:14% w/w (5% w:v of final lipid concentration)were dissolved in ethanol and mixed with an ethanol solution of the antioxidant α‑tocoferol (16 μM of final concentration). The lipid film was obtained through the evaporation of the organic solvent in a rotary evaporator at room temperature, at a pressure of 40 mbar and at the position 4 of the rotating speed control. To assure the elimination of residual traces of ethanol, the lipid film was left under high vacuum (Vaccuu brand GMBH+CO, VSP 3000- Germany) at least 3 h. The hydration of the dried lipid film was performed with 7% v:v ethanol in ultra-pure water and then the mixture was vortexed at 2400 rpm and above the phospholipid phase transition (-15°C for the heating curve and -7°C for the cooling curve) for 15 min, at room temperature. Afterward, the vesicles were incubated for 2 h at room temperature to swell and then extruded, at room temperature, ten times using a 10 mL lipex thermobarrel extruder (Northern Lipids Inc.) and polycarbonate filters of successive 0.2 and 0.1 μm pore size (Whatman Int. Ltd) to produce a homogeneous suspension. The increase of the extrusion number did not lead to better properties of the lipidic suspension in terms of size distribution. The incorporation of piroxicam in the deformable liposomes was performed by addition of an ethanol solution of the drug to the lipid solution or by the hydration of the lipid film with an aqueous solution of an inclusion complex of piroxicam with β-cyclodextrin. Piroxicam_β‑cyclodextrin complexes were prepared according the method used by Dalmora and Oliveira [22], with some modifications. Briefly, it was added an excess of piroxicam to an aqueous solution of β‑cyclodextrin (15 mM) and the pH was adjusted to 5.5 with citric acid. The mixture obtained was then shacked in a water bath at 25ºC for 24 h. The solution obtained after filtration with 7% of ethanol it was used to hydrate the lipid film. To fluorescent studies, was added to the water: ethanol solution or to the piroxicam_β‑cyclodextrin solution an ethanol solution of FITC (1 mg/ mL) in a proportion of 1:100 v/v. The separation of non-entrapped piroxicam and FITC was achieved by size exclusion chromatography using a Sephadex G-25 M column (GE-Healthcare). After pour off the column storage solution and to column equilibration it was used 25 mL of the solution used to hydrate the lipid film. Then, 2.5 mL of the liposomes suspension was putted onto the top on the column. The collection of the eluate was only made after put 3.5 mL of the solution to elute the sample that already entered the packed column completely. The low-molecular weight compounds were recovered with 25 mL of the same solution. | |
| Phospholipid quantification | |
| EPC was quantified by an enzymatic method (Lab Assay™ Phospholipid, Wako, Osaka, Japan) performed according to the manufacturer’s instructions. Briefly, phospholipase D hydrolyze phosphatidylcholine into choline. Then, choline is oxidized by choline oxidase into betaine. This reaction led simultaneously to the production of hydrogen peroxide. The reaction, catalysed by peroxidase, of this compound with N-ethyl-N-(2-hydroxy 3-sulfopropyl)-3,5-dimethoxyaniline sodium salt (DAOS) and 4-aminoantipyrine produced a blue pigment, which was quantified through the measurement of the absorbance at 600 nm in a multiplate reader (Synergy HT W/TRF from Bio-Tek). | |
| Liposomes characterization | |
| Size distribution: Particle size and polydispersity index (PDI) of the deformable liposomes (500 μM of EPC) was evaluated by dynamic light scattering (DLS) in a Malvern Zetasizer ZS (Malvern Instruments) equipment, at a scattering angle of 173º and at 25.0 ± 0.1ºC. The determination was performed at least three times for each vesicle samples (results expressed as mean value ± standard deviation). | |
| Morphology: The morphological characterization of liposomes was performed using a scanning electronic microscope model NOVA Nano SEM 200 FEI with a backscattered and secondary electrons detector. For this scanning transmission electron microscopy (STEM) analysis the liposome suspension was placed on copper grids with a carbon film 400 meshes and 3 mm diameter. The magnification was 50000× or 100000×. | |
| Entrapment efficiency: The concentration of piroxicam in the liposomes was determined by UV spectrophotometry (Heλiosγ ThermoSpectronic spectrophotometer; Unicam), at 354 nm, and subsequently it was possible to calculate the piroxicam entrapment efficiency applying the following equation: [28] | |
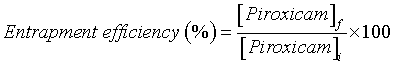 (1) (1) |
|
| where [Piroxicam]I and [Piroxicam]f is the concentration of piroxicam in the formulation before and after size exclusion chromatography. This calculation considers the NSAID concentration present in the deformable liposomes obtained after extrusion and in this way it was not affected by the vesicles loss that could occur during the preparation of deformable liposomes. The liposomes were destroyed using a solution of ethanol: water in a ratio of 3:1. | |
| Size stability: DLS was used to evaluate the size stability of the liposomes stored at 4ºC in a timeline of 2 months. The measurements of liposomes diameter were made for a period not superior to 10 days of difference between them. | |
| In vitro permeation of deformable liposomes: The in vitro permeation of empty deformable liposomes or entrapping piroxicam was evaluated through Franz diffusion cells (V-Series Stirrers for Franz Cells; Perme Gear, USA), presenting a diffusion area of 0.64 cm2. | |
| A preliminary study was performed with polysulfone membranes (50 nm of pore size) and then it was used abdominal pig skin. Pig skin is a good model for human skin permeability [29,30]. Membranes and pig skin were fixed between the donor and the receptor compartment. Abdominal porcine skin was excised from freshly slaughtered pigs at Central Carnes–Matadouro Central de Entre Douro e Minho, Lda. (Lousado, Portugal), following approved protocol by DGVDirecção Geral de Veterinária. Subcutaneous fat was removed and the skin washed with Phosphate Buffered Saline (PBS, pH 7.4). The integrity of skin was checked with a portable magnifying lamp before placing in Franz Diffusion Cells (PermeGear Inc., PA, USA) with the stratum corneum facing the donor compartment and the dermis facing the receptor compartment. The whole cells were placed onto a submersible stirrer plate set up in a water bath maintained at 37°C. | |
| After the acclimatization of the membrane and the tissue to the receptor phase (5 mL PBS, pH 7.4, thermostatically maintained at 37°C), the liposomal formulation (300 μL) was placed in the donor compartment. The buffer was continuously stirred and thermostated at 37ºC through a circulating water bath, to provide a skin surface temperature of 32°C by heat dissipation [31]. At determined time points (2, 4, 6, 8, 12 and 24 h) aliquots were taken and the same volume was replaced with PBS (pH 7.4). EPC concentration in the receptor compartment was assayed through the enzymatic method previously referred, by Vis spectrophotometry. At the end of the experiment the receptor phase was also collected to analyze the size and structure of the deformable liposomes. These analyses were made by DLS and STEM, respectively, as just referred. The skin surface, after 24 h of experiment, was washed with solutions presenting increasing ethanol percentages (50, 75 and 100%) to remove remaining donor sample. As the sample adsorbed to the skin is soluble in the solvents used to wash the skin, there was a complete removal of the remaining compounds. This approach is advantageous comparing with methods using aqueous solutions that acquire more than 99% of removal. Then, it was frozen for histological studies or used to determine the amount of phospholipid and FITC deposited in the skin (based in a published method [13] with some modifications). The skin explants were cut into small pieces and placed in a flask with 500 μL or 1 mL of ethanol. This mixture was incubated during 24 h with constant stirring to allow the ethanol diffusion through the skin to disrupt the deformable liposomes, releasing both the lipid and the liposomes content. The permeation percentage (or skin deposition percentage) was calculated by the following equation: | |
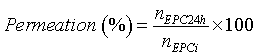 (2) (2) |
|
| Where nEPC24h is the n of phospholipid in the liquid receptor after 24 h of initializing the experiment and nEPC_i is the n of deformable liposomes with or without piroxicam_β‑cyclodextrin complexes placed in the donor compartment. | |
| Fluorescence microscopy studies: Vertical histological cuts (20 μm thickness) of frozen skin sections were made using a Leica CM1900 cryostat (Leica Microsystems, Numsloch, Germany). The distribution and degree of penetration of liposomes labeled with FITC into the skin was investigated in histological cuts by bright field and fluorescence microscopy - Leica DM 5000B Microscope (Leica Microsystems Numsloch, Germany) equipped with a DP72 digital camera (Olympus IX70, Hamburg, Germany). | |
| Statistical analysis: Data was reported as average ± standard deviation (all measurements were performed in triplicate). Statistical analysis was achieved by the Kruskal-Wallis One Way Analysis of Variance on Ranks (Sigma Plot 12.3). | |
Results |
|
| Liposomes characterization | |
| Piroxicam was entrapped in the lipid bilayer of deformable liposomes or in their aqueous compartment, through the use of β-cyclodextrin inclusion complexes, to evaluate which of the two approaches led to a higher entrapment efficiency of this NSAID. The piroxicam entrapment efficiency obtained when entrapped in the lipid bilayer was very low (3.15 ± 1.00%), as expected [25]. Thus, it was necessary to increase the aqueous solubility of piroxicam using β-cyclodextrin as referred. This procedure significantly enhanced the entrapment efficiency of piroxicam into deformable liposomes to a value of 19.93 ± 1.48% (63.27% higher than when piroxicam was entrapped in the lipid bilayer). As a result, for the further experiences it was used deformable liposomes containing piroxicam_β- cyclodextrin complexes. The mean size of the vesicles determined by DLS was 108.93 ± 3.74 nm (Figure 1) and the PDI presented a value of 0.07 ± 0.02. STEM analysis corroborated the results and showed that deformable liposomes were morphologically spherical (Figure 2). Measurements of liposomes diameter along two months of storage showed that the liposomes under study were stable, since there was no significative variation in size (Figure 3) and PDI along time. The PDI values were always lesser than 0.1. | |
| Figure 1: Size distribution of deformable liposomes (500 �?µM) containing complexes of piroxicam with �?�?-cyclodextrin. | |
| Figure 2: STEM microphotography of deformable liposomes containing complexes of piroxicam with �?�?-cyclodextrin at 50 000 x of magnification. | |
| Figure 3:Size (nm) measured after preparation and during two months of storage, at 4 °C, for deformable liposomes containing inclusion complexes of piroxicam with β-cyclodextrin. | |
| In vitro permeation of deformable liposomes | |
| Franz-type diffusion cells were used to estimate the permeation of empty deformable liposomes or containing the complexes of piroxicam with β‑cyclodextrin. Polysulfone membranes, which present a pore size smaller than the mean size of deformable liposomes, were firstly used to demonstrate that the deformable liposomes consisting only in EPC and sodium cholate or enriched with piroxicam_β-cyclodextrin complexes were able to reach the liquid receptor. The ability of each formulation to permeate the polysulfone membranes was equal to 44.25 ± 0.16% and 44.91 ± 2.13%, respectively, at the end of 24 h of experiment. In Figure 4, it is possible to observe the cumulative amount of phospholipid until the maximum of 24 h. It was also evaluated the vesicles permeation after 48 h of experiment, but the data obtained demonstrated that the fraction of deformable liposomes that crossed through the polycarbonate membranes did not conduct to a substantial increment of the permeation percentage of the empty deformable liposomes (44.9 ± 0.69%) and the liposomes with the piroxicam_β-cyclodextrin complexes (45.2 ± 1.23%). DLS analysis, performed to determine the diameter of the vesicles presented at the liquid receptor, demonstrated that deformable liposomes enriched or not with complexes of piroxicam with β-cyclodextrin presented a medium size of 90.22 ± 1.25 nm and 93.23 ± 1.32 nm, respectively. STEM analysis of the vesicles which permeate the polysulfone membranes revealed that the deformable liposomes had an average diameter nearly of the 100 nm and maintained the spherical shape (Figure 5). When the permeation studies were made with pig skin the deformable liposomes permeation decrease significantly. In fact, after 24 h of diffusion, only a percentage of 1.1 to 3.2% of the initial number of deformable liposomes was able to cross the skin structure reaching the receptor liquid. However, as showed by the histological studies, through fluorescence microscopy analysis (Figure 6), deformable liposomes were able to penetrate deeper into the skin. Figure 6 shows that, despite the higher fluorescence intensity at the stratum corneum, the deformable liposomes were nearly distributed uniformly on the skin structure. Moreover, the percentage of phospholipid deposited in the pig skin, after 24 h of incubation with ethanol, was 1.04 ± 0.05% or in terms of mass/area was equal to 0.20 ± 0.01 mg/cm2. The percentage or amount/area of FITC that remained in the skin after the experiments provided a value of 1.03 ± 0.21% or 6.70 + 0.97 μg/ cm2, respectively. Comparing the mean molar percentage of EPC and FITC it is possible to verify that they were similar, which means that they were retained in the same proportion relatively to the quantity applied on the skin. | |
| Figure 4: Cumulative amount of EPC (g/cm2) in the liquid receptor after polysulfone membranes permeation of deformable liposomes with or without piroxicam_β-cyclodextrin(CD) complexes, for 24 h, at 37°C. | |
| Figure 5: STEM microphotographs of deformable liposomes without (a) or with (b) piroxicam_β-cyclodextrin complexes after the permeation studies with polysulfone membranes (50 nm of pore size), at 100 000x of magnification. | |
| Figure 6: Fluorescence microscopy images of the penetration of FITC labeled deformable liposomes without (a and c) or with (b and d) piroxicam_β- cyclodextrin complexes into the pig skin, after 24h of incubation. | |
Discussion |
|
| In this work, piroxicam was entrapped into deformable liposomes to be delivered through the skin in order to treat inflammatory diseases. Two approaches were used to entrap piroxicam into the liposomes formulation. The first involved the inclusion of this NSAID in the lipid bilayer. However, the entrapment efficiency obtained was low (3.15 ± 1.00%) and significantly different from data present in the literature for piroxicam in EPC liposomes [32]. In that work it is also possible to conclude that the increase of the lipid concentration led to a higher incorporation of piroxicam. This behavior was not verified in this study, once that it was used a higher lipid concentration. This difference can be due to the presence of sodium cholate in the deformable liposomes composition, which it is also associated with the lipid bilayer and consequently can lead to a reduced ability of piroxicam incorporation. A similar result was obtained for the hydrophobic drug betamethasone [7]. Thus, it was necessary to increase the aqueous solubility of piroxicam using β-cyclodextrin. This procedure significantly enhanced the entrapment efficiency of piroxicam into deformable liposomes (63.27% more than in the first approach) and, consequently, was the procedure assumed for the subsequent assays, as verified in other works [24,27]. The preparation method of deformable liposomes led to a value of PDI smaller than 0.1, which means that the vesicles population was homogeneous in size (≈ 100 nm) even two months of storage [33]. The stability of the spherical deformable liposomes was also confirmed in terms of size, as there was no significative variation in this parameter along time. To estimate the permeation through the skin of the deformable liposomes with or without complexes of piroxicam with β‑cyclodextrin it was used Franz-type diffusion cells. First, it was used polysulfone membranes to demonstrate that the developed deformable liposomes present enough elasticity to pass through these membranes with a pore size smaller than their diameter, as observed by other authors [27]. Their ability to permeate the polysulfone membrane was significative after 24h. Comparing the size of the deformable liposomes that reach the liquid receptor (≈ 90 nm by DLS) with the values obtained after preparation it is evident that there was a reduction on the mean diameter of deformable liposomes, which means that the smaller particles were able to permeate more easily. Additionally, it was possible to conclude that the spherical shape was maintained after passage through the polysulfone membranes. These results support the good deformability behavior of the prepared deformable liposomes. The decrease of permeation obtained when the assays were made with pig skin is directly related with the expected role of the stratum corneum in counteracting xenobiotics penetration into the skin, which is not considered in the membrane assays. Despite the formidable barrier provided by the stratum corneum, deformable liposomes were able to penetrate deeper into the skin, as showed by the histological studies. In fact, the distribution of green colour provided by FITC incorporated into deformable liposomes was uniform through all skin structure. Indeed, it has been suggested that in association of the penetration enhancing effect, deformable liposomes should be intact to increase the drugs skin permeation [16]. Concluding, in this work it was used deformable liposomes to deliver piroxicam through the skin. Therefore, optimization of vesicles preparation and characterization was followed by in vitro permeation experiments to demonstrate the ability of the deformable liposomes to be used in topical applications, as to penetrate deeply in the skin layers. The studies performed with polysulfone membranes demonstrated that the vesicles under study presented enough elasticity to pass through smaller pores than liposomes medium size. Furthermore, DLS and STEM analysis showed that deformable liposomes maintain the size and morphology after pores penetration. Permeation experiments with pig skin, demonstrate the ability of these vesicles to deliver piroxicam even to the deepest skin layers. The results support the use of deformable liposomes as suitable topical vehicles to deliver piroxicam for the treatment of inflammatory diseases. | |
Acknowledgements |
|
| The authors would like to thank Matadouro - Central Carnes de Entre Douro e Minho, Lda for their availability to provide pig skin, the Department of Histology from Life and Health Sciences Research Institute (ICVS) of University of Minho for the assistance given in the histological studies and the Department of Biology of University of Minho for the fluorescence microscopy analysis. | |
Declaration of Interest |
|
| Helena Ferreira would like to thank POPH/FSE for co-financing and FCT for fellowship SFRH/BPD/38939/2007. The authors declare no conflicts of interest. | |
References |
|
|
|

