Research Article, J Pharm Drug Deliv Res Vol: 4 Issue: 2
Comparative Study of a Drug Release from a Textile to Skin
| Cezar Doru Radu1*, Oana Parteni1, Marcel Popa2, Ioan Emil Muresan2, Lacramioara Ochiuz3, Laura Bulgariu2, Cornel Munteanu4, Bogdan Istrate4 and Eugen Ulea5 | |
| 1Department of Textiles, Leather and Industrial Management, Gheorghe Asachi Technical University of Iasi, Romania | |
| 2Department of Chemical Engineering and Environmental Protection Mangeron, Gheorghe Asachi Technical University of Iasi, Romania | |
| 3Department of Pharmacology, University of Medicine and Pharmacology of Iasi, Romania | |
| 4Department of Mechanical Engineering, Gheorghe Asachi Technical University of Iasi, Romania | |
| 5Department of Plant Science, I. Ionescu Brazi University of Agricultural Science and Veterinary Medicine, Romania | |
| Corresponding author : Cezar Doru Radu Department of Textiles, Leather and Industrial Management, Gheorghe Asachi Technical University of Iasi, Romania Tel: +40748830254; Fax: +40 232 230491 E-mail: rcezar2010@yahoo.com, oanaparteni@yahoo.com |
|
| Received: September 08, 2015 Accepted: October 13, 2015 Published: October 20, 2015 | |
| Citation: Radu CD, Parteni O, Popa M, Muresan IE, Ochiuz L, et al. (2015) Comparative Study of a Drug Release from a Textile to Skin. J Pharm Drug Deliv Res 4:2. doi:10.4172/2325-9604.1000134 |
Abstract
Comparative Study of a Drug Release from a Textile to Skin
Objective
The achievement of a therapeutic dose of the hydrocortisone acetate (HCr) for coetaneous affections by using: i) a cyclodextrine derivative and ii) a hydrogel, in the systems fixing the drug on a textile material and then releases it in vitro towards the dermis under the action of a perspiration kit.
Methods
The formation of a temporary deposit of HCr on a cotton knitted fabric in the variants: 1) covalent grafting with monochloro triazinyl-beta-cyclodextrine (MCT-β-CD) by complexation in the hydrophobic cavity of the CD; 2) through the inclusion of Na2SO4 in ionic crosslinked chitosan-based hydrogel. Upon the release of HCr in vitro, a perspiration kit has been used.
Results
The two medicine release systems can release the therapeutic dose for coetaneous disorders.
Conclusion
The comparison of the two systems improves the understanding of the release mechanisms which may provide useful insights when designing medical textiles.
Keywords: CD: Cyclodextrine; CS: Chitosan; HCr: Hydrocortisone acetate; MCT-β-CD: Monochloro triazinyl-β-cyclodextrine; NADPH: Nicotinamide adenine dinucleotide phosphate-dehydrogenase
Keywords |
|
| Cotton knitted fabric; Monochloro triazinyl-β-CD; Hydrogel; Chitosan; Therapeutic dose; Coetaneous disorder; Drug release | |
Abbreviations |
|
| CD: Cyclodextrine; CS: Chitosan; HCr: Hydrocortisone acetate; MCT-β-CD: Monochloro triazinyl-β-cyclodextrine; NADPH: Nicotinamide adenine dinucleotide phosphate-dehydrogenase | |
Introduction |
|
| Multifunctional textiles worn straight on skin (blouse, trousers, and pants) have the role of thermal protection and coetaneous comfort; they can also have a therapeutic role through a drug release from the knitted fabric to the skin, when the textiles are made of medical fabric. The drug release occurs for a time period which depends on the fabric loading degree and it is triggered by a series of physiological factors, like: enzymes (i.e. nicotinamide adenine dinucleotide phosphatedehydrogenase (NADPH), Cytochrom P450, esterases, amidases, gluthation S-transferase, methyl transferase, acetyl transferase), hydro-electrolytic secretion of the sweat glands with a slight acid pH (coetaneous pH=5.5) that can favor the drug release through diffusion, body temperature and skin friction with the fabric [1]. For the chemical modification of the fabric surface there are different alternatives which permit to generate the drug reservoir on the textile support: a) micro-emulsions [2]; b) hydrogels [3] and c) CDs [4-7]. When micro-emulsions are used, the drug transfer to the dermis and diffusion to the systemic level implies generation of permeation channels through the dermis corneous layer. Hydrogel deposits on the textile surface, the drug being released by a typical diffusion process. Numerous CD applications are based on the complexing capacity of lipophilic molecules with molecular size adequate to the cavity inner diameter. In literature, one can find communications concerning the CD use in both medical textiles [8,9] and other applications [10-12]. Among the polysaccharides able to form hydrogels on the fabric support, CS, due to its both ionic and covalent (or combined) crosslinking possibility and its anti-microbial character, is used in various pharmaceutical and medical applications or as antimicrobial agent when applied on textile fabric [13,14]. Drug diffusion from fabric to the skin occurs as long as the coetaneous stimuli favor the transfer from the textile surface to dermis. In this case, diffusion occurs without conscious patient intervention on the drug. Drug transfer occurs as long as the fabric in contact with the human epidermis still contains an amount of drug. This aspect already proves the advantage of medical textile use. | |
Medication |
|
| Regardless the therapeutic class to which they belong, drugs present structures which confer them either a lipophilic or a hydrophilic character. Lipophilic molecules or structural segments are absorbed inside the CD hydrophobic cavity, the process being encouraged by low temperatures and relatively long time periods. The controlled drug release systems that use CD-grafted textiles have been studied for various therapeutic applications: chronic venous insufficiency [4], allergic dermatitis [15], psoriasis [16-18] and microbial infections, HCr is the main physiological glucocorticoide utilized as such or as esters. From a pharmacy-dynamic standpoint, HCr has an anti-inflammatory action of reference for glucocorticoids. Figure 1 illustrates the HCr structure. At present, HCr is used in therapeutics for its anti-inflammatory, anti-allergic and anti-itching effects, having applications in psoriasis treatment too. The classical formulations are: injectable solution (25 mg/mL), ointments, creams, emulsions, suppositories, lotions with concentrations ranging within the interval 0.25%-2.5%. For topical applications, the quantity varies from 1.7 g ointment/cm2 skin when taken from a jar, to 0.7 g unguent/cm2 skin, when taken from a tube [19,20]. The goal of this study is to achieve two types of polymer-drug systems with controlled or sustained release, having as support a textile material modified in two different ways, in order to permit the subsequent association of HCr. The application considered by the authors is on knitted textile with potential applications in psoriasis treatment; as far as we know, no such system has been reported in literature. A first system is realized by chemically grafting CD (under the form of MCT –β-CD) to the polysaccharide chain of cotton, followed by HCr inclusion in hydrophobic CD cavity. The second one is realized by the deposition of a hydrogel based on ionic cross-linked CS on a knitted fabric surface, simultaneously with HCr inclusion. The latest procedure is an original contribution and it has not been communicated in the specialty literature. Taking into account that HCr is at present formulated in topical compounds with concentrations ranging between 0.5- 2.5%, we produced in our study a drug loading of 5 mg hydrocortisone/ 25 cm2 of textile material (square sample of cotton, sized 5×5 cm, with the weight of 0.5 g). The realized loading degree is situated within HCr therapeutic interval and permits the administration/contact with an extended skin surface with minimum risks of apparition of systematic secondary effects. From the information mentioned above, it follows that the degree of loading with HCr was of 10 mg HCr/g textile material. The work proposes a comparative study of the kinetics and efficiency of HCr release in time from the textile material, for the two ways of biomaterial realization. | |
| Figure 1: HCr structure. | |
Materials |
|
| The textile material consists of a 100% cotton interlock knitted fabric commonly used for manufacturing pajamas, body blouse, under vests. The knitted is manufactured from cotton yarns with yarn count, Nm= 60/l. MCT-beta-CD type Cawatex is purchased from Wacker Chemie; Lavotan DSU, surfactant provided by Clariant, is a wetting, emulsifying and cleaning agent for all types of fibers; Sirrix 2UD is an anionic product in the form of organic acid mixture, provided by Clariant, which has a de-mineralizing action of cellulose fibers; Na2S2O3 is used as reducing agent to prevent cellulose degradation during boiling under the action of oxygen from alkaline solutions; Contavan ARL is a stabilizer of hydrogen peroxide consisting of a surfactant mixture supplied by Bezema; CS with a de-acetylating degree of 75-85%, with molecular weight ∼ 600 kDa, delivered by Fluka as highly viscous CS form; HCr purchased from pharmaceutical market, as a white powder, was used without modifications; Ferric chloride, potassium hexacyanoferrate, sulphuric acid and all other reagents (NaOH, Na2CO3, H2O2, Na2S2O3) were used without modifications as purchased from Sigma Aldrich supplier. | |
Methods |
|
| After knitting, the textile fabric is subjected to the following treatments presented in Protocol 1: 1) Alkaline boiling at a liquor ratio of 1:1, using 10% NaOH (reported to the textile material weight), 5% Na2CO3, 1 g/L Lavotan DSU, 1 g/L Sirrix 2UD and 2 g/L Na2S2O3; 2) Alternative washing for 10 min. at 90oC and 10 min. at 20°C; 3) Bleaching with hydrogen peroxide (through pad-batch, at a liquor ratio of 1:20, using: 20 ml/L H2O2 (32%), 20 g/L NaOH, 5 g/L Lavotan DSU and 4 g/L Contavan ARL, and then storage at room temperature for 18 hours; 4) Repeated warm and cold washing operations; 5) Drying 24 h at room temperature. MCT-β-CD grafting on knitted fabric was carried out through pad-dry-cure procedure, using the working protocol 2. | |
| Protocol 2: 1) Padding with a solution of 100 g/L MCT-β-CD and 20 g/L Na2CO3 at a squeezing degree of 135% (100 kg textile material contains 135 liters treatment solution); 2) Drying at 80oC, 10 min.; 3) Curing at 160°C, 7 min.; 4) Repeated warm and cold washing to remove the reaction products until a pH=7 is obtained; 5) Final drying at 80°C, 10 min. | |
| HCr absorption on textile grafted | |
| A solution of 0.06 g HCr in 100 ml ethyl alcohol was prepared. From the solution 20 ml was extracted in which the fabric was immersed (sized 5×5 cm) for 24 h at room temperature under magnetic stirring (400 rpm), after which the fabric was first dried at room temperature and then at 40°C for 5 h. The solution remained after removing the sample was filtered, and then the amount of unabsorbed HCr was spectrally determined. Making the difference from the drug amount in the initial solution, the HCr amount remained on the fabric is determined. | |
| Dosing the HCr | |
| HCr dosing through UV spectroscopy did not lead to conclusive results, which imposed an analytical solution [21,22] based on HCr oxidation to the acid form, based on the reduction of ferric salt to ferrous salt, with the formation of an yellow solution; the color is intensified with potassium hexacyanoferrate to a bluish-greenish shadow [21]. The scheme of the redox system is presented in the Equations 1-3. | |
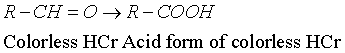 (1) (1) |
|
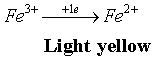 (2) (2) |
|
| A HCr-based solution of 60 mg/100 ml ethyl alcohol was prepared. The solutions for HCr calibration curve contain 2 ml sulphuric acid (4N), 2 ml ferric chloride solution (0.5% v/v), 0.5 ml potassium hexacyanoferrate (0.5% v/v), 3 ml perspiration kit, and increasing concentrations of HCr from the basic solution (0.5; 1.0; 1.5 and 2.0 ml); a witness solution which contains 2 ml H2SO4 (4N), 0.5 ml potassium hexacyanoferrate (0.5% v/v), 3 ml perspiration kit and 2 ml ferric chloride (0.5% v/v) is added to these. The prepared solutions are thermostated 15 min at 70°C, then cooled down and brought at constant volume of 25 mL with distilled water. The absorbance is determined versus witness sample at 780 nm, finally plotting the calibration curve. | |
| HCr quantity released from textile grafted | |
| HCr release in vitro was performed using a perspiration kit according to ISO 105-E04-2008 standard [23]. The textile material is immersed in the perspiration kit solution which contains 0.05% (w/v) L histidine monohydrochloride monohydrate, 0.5% (w/v) NaCl and 0.22% (w/v) NaH2PO4·2H2O (23) at a liquor ratio of 1:40 (0.5 g textile material in 20 ml kit) at 37°C. At pre-established storage durations (1, 3, 6, 12 and 24 h) at 37°C, were stored the solutions in which the textile samples were immersed and the amount of HCr following the previously described method was determined, the amount of released HCr was spectrophometrically dosed. To draw the calibration curve for the concentration of HCr in the alcohol solution in hydrogel a solution of 100 ml 25% ethyl alcohol (v/v) is prepared and then 0.06 g HCr dissolved in it. By measuring the absorbance corresponding to the serial dilutions the calibration curve was plotted. The dosage used the analytical method described by reactions (1)-(3). | |
| Deposition of CS-based hydrogel on textile | |
| A CS solution is prepared in which the knitted fabric samples are immersed at a knitted fabric/CS ratio of 1:1 (w/w). 0.5 g CS is solved in 50 ml distilled water in Berzelius beaker, warm, slightly stirring in acid medium of glacial acetic acid (96%). After 12 h, the samples are removed from solution and fastened on a device hanging over the beaker from which pours the solution to be impregnated in the knitted fabric. CS saturated knitted fabric is immersed in a solution of HCr and cross-linking agent (Na2SO4) for 24 h, under stirring at the environmental temperature. The drug solution was prepared by dissolving 0.06 g HCr in 100 ml 25% ethyl alcohol. In the solution was added 0.156 g Na2SO4. From the solution was taken 20 ml to include the fabric which already had CS. The amount of Na2SO4 was calculated as related to CS acetylation degree, in order to provide a slight excess as compared to the number of moles of ammonia group with which it forms ionic bonds. The final ethyl alcohol/water ratio is 25/75%. | |
| The HCr released from textile with hydrogel | |
| The used protocol is the following. The sample with hydrogel deposited on its surface containing the incorporated drug is immersed in a volume of 20 ml perspiration kit. After 1 hour the samples were removed from the kit solution and dried. Then they were introduced in the solution of perspiration kit for 3, 6, 12 and 24 hours. | |
| Determination of textile swelling | |
| Due to the reduced solubility of HCr in water (1 mg/100 ml H2O; 0.45 g/100 ml ethyl alcohol), a 25% ethyl alcohol solution was used as swelling medium. The time evolution of the swelling degree of the knitted fabric containing CS hydrogel deposited in its surface as compared with the initial knitted fabric. An amount of 0.3674 g knitted fabric was introduced in 50 ml solution of ethyl alcohol (25%) at time durations of 1, 3, 5, 10, 20, 30, 50 and 60 minutes. After this time, the knitted samples are removed from the solution, slightly dabbed with filter paper to remove the excess solution, and then weighted. The swelling degree (SD) is expressed in percents based on the Equation 4. | |
| Where: Mf is material’s weight after swelling; Min=weight of initial material (dry). | |
| Evaluation of textile material loading degree | |
| The material degree of loading either with MCT-β-CD or with CS is determined by accurately weighting (10-4g) the textile using the following expression (5): | |
| where Mf=final textile weight after loading (g); Mi=weight of initial textile. | |
| Absorbance determinations for the solutions containing HCr for the both systems, MCT-β- CD and CS-HCr were carried out using the UV-VIS spectrophotometer KJ- VS-721N Shanghai Jing xue, China, 1999, at the wavelength of 780 nm. We have used AMETEC EDAX equipment for elemental analysis, coupled with SEM Quanta 302D and Genesis Software. An oven type Venticelly 55, 2009 is used to maintain the temperature constant up to 100°C. The thermal treatments performed on the knitted fabric were carried out on Mesdan Lab Dryer 2008 within the range 20-180°C. Hettigh Zentrifugen Universal 320 R was used to centrifuge the solutions. For the quantitative nitrogen determination we used a HNS-O Analyzer, Flash EA, 112 Series, 2010, the combustion method combined with chromatography in gaseous phase with a TC detector. | |
Results |
|
| The results obtained after performing the studies are illustrated in two sections; dedicated to each of the two types of resulted biomaterials. | |
| Textile grafted and loaded with HCr | |
| A first stage of our research consisted in the creation of the conditions for drug association to the textile support by chemically grafting the MCT-β-CD to the cellulose of cotton knitted. The textile material was prepared for experiments using the protocol 1. MCT-β- CD grafting to cotton knitted cellulose was performed according to the protocol 2. The chemically modified material obtained in this way was at first characterized from structural and morphological standpoints. Figure 2 illustrates the weight of chemical elements (relative units) from the knitted fabric surface grafted with MCT-β-CD, estimated through EDAX elemental analysis. Namely, the nitrogen presence (resulted from the elemental analysis in Figure 2) on the surface of cellulose fibers indicates the presence of triazinyl group from MCT- β-CD on cellulose. The presence of other elements, such as: Na, Al, Si, Cl, Ca, etc is due to final washing of the cotton knitted fabric with running water, as the preparation was made on a large textile material quantity. The morphology of the fibers from the modified material is illustrated in Figure 3, in comparison with witness knitted fabric. From the images presented in Figure 3A and 3B, it is noticed that by grafting MCT-β-CD new irregular morphological formations appear, with quasi-polygonal shape and variable dimensions (from 1.07 to 4.99 μm), which represent the support of temporary drug deposits. The micrograph from Figure 4A reveals the morphological aspect of the knitted fibers grafted with MCT-β-CD and complexed with HCr, as compared to the textile, from which the drug was partially released in the perspiration kit B. The results of drug release are illustrated in Figure 5, which represents the kinetics of HCr release from cotton surface grafted with MCT-β-CD. The perspiration kit was used at pH=5.5, identical with the pH of human dermis, and temperature of 37°C (physiological temperature of body) for modeling the physiological coetaneous conditions of drug release) Equation 5. | |
| Figure 2: EDAX elemental analysis of the knitted fabric grafted with MCT-Β-CD. | |
| Figure 3: SEM microphotography: A) knitted fabric (witness); B) knitted fabric grafted with MCT-Β-CD. | |
| Figure 4: SEM microphotography: A) knitted fabric grafted with MCT-ß-CD and complexed with HCr; B) knitted fabric after drug release. | |
| Figure 5: Kinetics of HCr release from the knitted fabric grafted with MCT-Β-CD. | |
| In order to estimate the release characteristics of a drug from a polymeric structure, several mathematical models have been proposed, among which Korsmeyer-Peppas [24-26] is frequently used. Equation 6 represents Korsmeyer-Peppas relation: | |
| Where: Mt is the quantity of drug released at the moment t, M∞ is total quantity of included drug, k is the release constant and n is the release coefficient. For determination, one needs to analyze the segment of curve of the HCr release from textile surface for which the release is smaller than 60%. The coefficient n is determined by processing the experimental data Mt, M∞ and t. The experimental values of the exponent taken from literature [24] can provide information on the drug transport and release mechanisms. The value of the release coefficient Equation 6 is n= 0.79, situated in the non- Fick diffusion range. | |
| Biomaterial with hydrogel deposited on textile | |
| The hydrogel-knitted fabric system is characterized from structural and morphological standpoints, as well as in terms of its swelling capacity in physiological liquid, an important characteristic for the drug release systems controlled by diffusion. Figure 6A presents the aspect of native cotton fibers and Figure 6B presents the morphology of knitted fabric fibers after the deposition of the gel based on ionic cross-linked CS. In Figure 6B one can notice that hydrogel forms a matrix in which the cotton fibers are immersed. Figure 7 confirms the CS presence in the hydrogel deposed on textile material, by the presence of nitrogen originating in the amino group, and of the ionic cross-linking agent (Na2SO4), through the presence of sulphur, demonstrated by the elemental analysis. Since the hydrogel swelling characteristics in aqueous mediums are determining factors for the amount of included/released drug, as well as for drug transport/release mechanisms, we proceeded to the assessment of the swelling kinetics of the new materials in such mediums. Given the fact that HCr inclusion was realized in an aqueous solution of ethyl alcohol (25%), the swelling process was studied in this medium. Figure 8 illustrates the swelling kinetics of the knitted fabric, while Figure 9 presents the swelling kinetics of cotton knitted fabric with hydrogel. The kinetics of HCr release from hydrogel is illustrated in Figure 10. | |
| Figure 6: SEM microphotography of cotton fibers, A) Before hydrogel application; B) After hydrogel application. | |
| Figure 7: EDAX elemental analysis of the knitted fabric with CS-based hydrogel. | |
| Figure 8: Swelling kinetics of knitted fabric in an (25%) ethyl alcohol solution. | |
| Figure 9: Swelling kinetics of knitted fabric with hydrogel in an ethyl alcohol (25%) solution. | |
| Figure 10: Kinetics of HCr release from knitted fabric with hydrogel. | |
| The experimental value of the release coefficient obtained from Korsmeyer-Peppas in Equation 6 is n = 1.03. | |
Discussion |
|
| Biomaterial with CD derivative | |
| In order to confirm the presence of the CD triazine derivative, the EDAX elemental analysis is frequently used in 2nd Figure. In the case of a cotton fabric, nitrogen presence certifies the presence of the azine group on the cellulose. However, nitrogen confirmation does not justify the formation of the covalent linkage between the CD derivative and the cellulose; the triazine compound can be physically adsorbed on the surface of the cotton material and then it can be quantitatively determined. Therefore, in order to confirm the grafting on the cellulose we compared the FTIR-ATR spectra of the thermally cured samples with MCT-β-CD at 160°C to the control samples, visualizing the modifications of the IR absorption bands at the wavelengths of 874 and 810 cm-1 characteristic to the triazinyl groups. Given that a large quantity of cotton knitted fabric was grafted with MCT-β-CD, the results were communicated elsewhere [27]. For the experimental accuracy, we added the results obtained in the nitrogen quantitative elemental analysis from the textile samples, through the method of the coupled combustion with chromatography in gaseous phase. The results are presented in Table 1, where the presented values are the average of four determinations. | |
| Table 1: The nitrogen content percentage of the tested samples. | |
| The data presented in Table 1 confirm together with the FTIRATR spectra MCT-β-CD presence on the cellulose. Having the certainty that CD derivative is grafted on the textile material, we consider that between cotton cellulose and the triazine derivative a nucleophilic substitution reaction takes place, as illustrated in Equation 7. | |
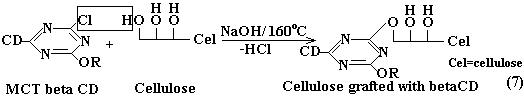 |
|
| By examining the SEM microphotography, we can notice the occurrence of certain morphological formations disposed according to the microphotography in 3B figure and attributed to MCT-β-CD. In Figure 4A, the appearance of the grafted surface combined with HCr is more obvious than the formations in Figure 3B. But the oval shape of the formations seen on the fibers surface in Figure 4B can imply the existence of a damaging action exerted by the perspiration kit on the knitted fabric. It seems that the kit perspiration has a detersive action on temporary reservoirs of HCr. According to the determinations performed by analytical method, from the initial solution of 24 mg HCr, the textile material absorbed 10.04 HCr/g of knitted fabric. This means that at a sample with the mass of 0.5 g cotton, the amount of HCr is 5.02 mg/0.5 g cotton, a value representing practically the therapeutic dose. One mentioned the value represents the drug amount absorbed. After HCr release from the surface of the grafted textile material, the calculations performed through the analytical method present the following situation. Since the therapeutic doses for topical applications (cream, gel) is of 5 mg HCr/0.5 g textile material, it is considered that, under working conditions, the concentration of released drug is near the therapeutic doses for topical applications. The argument is represented by the amount of HCr released (4.75 mg) for a knitted fabric mass of 0.5 g; namely, one can consider that the experiment is in the proximity of the therapeutic doses range. Drug-release efficiency of drug release is of 94.6% for 24 h duration. Efficiency was calculated as the ratio between the released drug amount and the drug adsorbed amount = 4.75/5.02 = 0.94. Only theoretically, by extrapolating the kinetic curve from 5th Figure, one can estimate the maximum duration for 100% HCr release, which is about 37.5 h. The calculating procedure is not presented in this work, but it supposes the extension of the last right portion of the diagram in 5th Figure, until the straight line segment intersects the value of 100% of the released HCr; in this point, we must read on the abscissa the appropriate value of the duration (37.5 hours). HCr was applied without any discrimination on the knitted fabric surface; at HCr release, one does not know the weight of HCr complexed inside β-CD cavity, and the HCr amount released from the interstices of the knitted fabric. Even if the above mentioned aspect can be experimentally solved, it was not considered in this work. The kinetics of HCr release 5th Figure, presents an exponential character typical for the systems of controlled release of drugs bound on polymeric supports. | |
| In case of the Korsmeyer-Peppas (Equation 6), the experimental value of the release coefficient n = 0.79 represents a measure of the hydrophobic interactions between inner cavities of the CD and the functional groups of HCr. Drug displacement from cavities occurs under the action of the perspiration kit solution. | |
| Biomaterial with hydrogel | |
| In principle, the formation of CS-based hydrogel deposited on knitted fabric surface is possible as the result of ionic crosslinking with Na2SO4. In acidic conditions, the CS amino groups are protonated, such that strong ionic interactions between Na2SO4 and polysaccharide become possible. Schematically, the hydrogel structure is as follows: As a main, a stronger cross-linking can be realized through covalent bonds, but cross-linking agents of this type (glutaraldehyde, epichlorohydrin) are toxic and are not indicated for the production of hydrogels destined to biomedical applications. Hence the utilization of Na2SO4 is perfectly justified. | |
| From the 8th and 9th figures it is noticed that after keeping the knitted fabric for 60 min. in alcohol solution, it reaches a swelling degree of 124%, while for the knitted fabric with hydrogel a value of 189% is obtained. The difference between the two values 189-124=65% represents the swelling value due to the hydrogel presence on the knitted fabric. One can also notice that the swelling rate during the first minutes of the process is higher in the case of the knitted fabric with hydrogel deposited on the textile material. Water molecules diffuse among the CS macromolecular chains and break the inter-chains hydrogen bonds, replacing them with hydrogen bonds established between CS and water. The remarkably high swelling capacity in these conditions permits the conclusion of a high potential of drug sorption in hydrogel. In the case of the HCr inclusion in the hydrogel and the subsequent release, we obtained the following experimental situation, resulted from the analytical method of medicine determination. The initial quantity of HCr in solution was 24 mg HCr, while what remained in solution after removing the textile material is of 10.50 mg HCr/g material; therefore there is a quantity of HCr absorbed by 13.5 mg HCr/g textile material, and 6.75 mg absorbed HCr/0.5 g textile respectively. In Figure 10 (Kinetics of HCr release from knitted fabric-hydrogel system) too, one can notice that the process kinetics is typical for diffusion systems of the polymeric support-drug type, with a quite marked burst effect during the first four hours of the process. On the other hand, it is quite clear that the release rate is high, being practically total after about 24 h; namely, out of the 6.75 mg HCr/0.5g of knitted fabric fixed on the textile support, 6.15 mg HCr/0.5 g of knitted fabric are released. The efficiency of drug release is of 91.1%. Based on the experimental value of releasing coefficient, n=1.03 calculated according with Korsmeyer-Peppas (Equation 6) it is obvious that drug transport and release through the system are not governed only by diffusion, the process mechanism being probably disturbed by hydrogel erosion under the action of the salts from the perspiration kit, which can destroy in time the ionic cross-linking. Above the process of HCr release from hydrogel, the process of drug release from the cotton fiber is superposed, as the cotton fiber is able to absorb reduced drug amounts. The pH of perspiration kit is 5.5, close to skin pH. The cationic compounds of the kit modify ionic balance of released solution. A hypothetical structure of hydrogel is illustrated in Figure 11. The image in Figure 11 shows a polymer structure under the shape of loops, formed on the opposite sides by two fragments of CS closed through ionic interactions between the positive groups of the CS amine and the negative sulphate groups of Na2SO4. Therefore, besides the appropriate swelling capacity, the polymer configuration of hydrogel favors the creation of certain drug sorption spaces. On the other hand, in the paper, it was not possible to assess the drug absorption type, mono/multilayer, in the hydrogel matrix. | |
| Figure 11: The hypothetical structure of a CS-based hydrogel. | |
| Other considerations | |
| By comparing the two systems, one can appreciate that the therapeutic potential of the hydrogel of CS is higher due to the additional antimicrobial action of the amine group of polysaccharide and the corresponding compatibility with human skin. Yet, the CS fixed on textile material has a weak hold further to repeated washing, unlike the CD derivative grafted on cellulose, which has a strong hold. Therefore, for CS-HCr system it is imperiously necessary to optimize the factors which intervene in the adsorption of an adequate drug amount in the hydrogel matrix. The proposed drug-release systems have a specific behavior. Namely, the CDs are adequate for the release of lipophilic drug whose bio-availability at tissue level it will amplify [28,29]. Similarly, the CS hydrogel has a good capacity to enclose the drug, whose release only depends on pore size, or the density of crosslinked structure through which it diffuses to the polymer mass exterior. The comparative study of the two systems meant to form temporary drug deposits on the knitted fabric highlights the differences in terms of the way of drug inclusion (complexing within CD and hydrogel generation as a matrix for HCr inclusion respectively); yet, the drug release mechanism is relatively similar. In the case of CD, the perspiration kit creates conditions for the substitution of HCr from cavity and its transfer to dermis; for hydrogel, the kit is the medium for swelling the structure and implicitly for carrying the drug through the CS hydrophilic mesh, with the effect of drug diffusion toward dermis. | |
Conclusion |
|
| The systems of HCr release from MCT-β-CD and the CS-based hydrogel can represent support-structures for drug release from the knitted fabric worn directly on skin for a coetaneous therapy. Both systems have a corresponding potential of application, even if they can be optimized strictly in terms of the HCr controlled release characteristics at the topic level. Drug release for the investigated systems has a burst effect. The advantage of using the drug release systems is due to the adequate bio-compatibility of CD and CS, products obtained from natural raw materials, as well as to the improvement of physical-chemical properties of lipophilic drugs that need to increase their dispersion capability in biological mediums, as well as to the realization of some therapies from a textile structure, which diminishes the patient contribution and conscious effort in accomplishing the drug administration procedure. | |
Acknowledgments |
|
| The work was supported by the strategic grant POSDRU/159/1.5/S/133652, co-financed by the European Social Fund within the Sectorial Operational Program Human Resources Development 2007-2013. | |
References |
|
|
|

